Scientists Have a Plan to Hunt the Ancient, Dead Star that Birthed Our Solar System
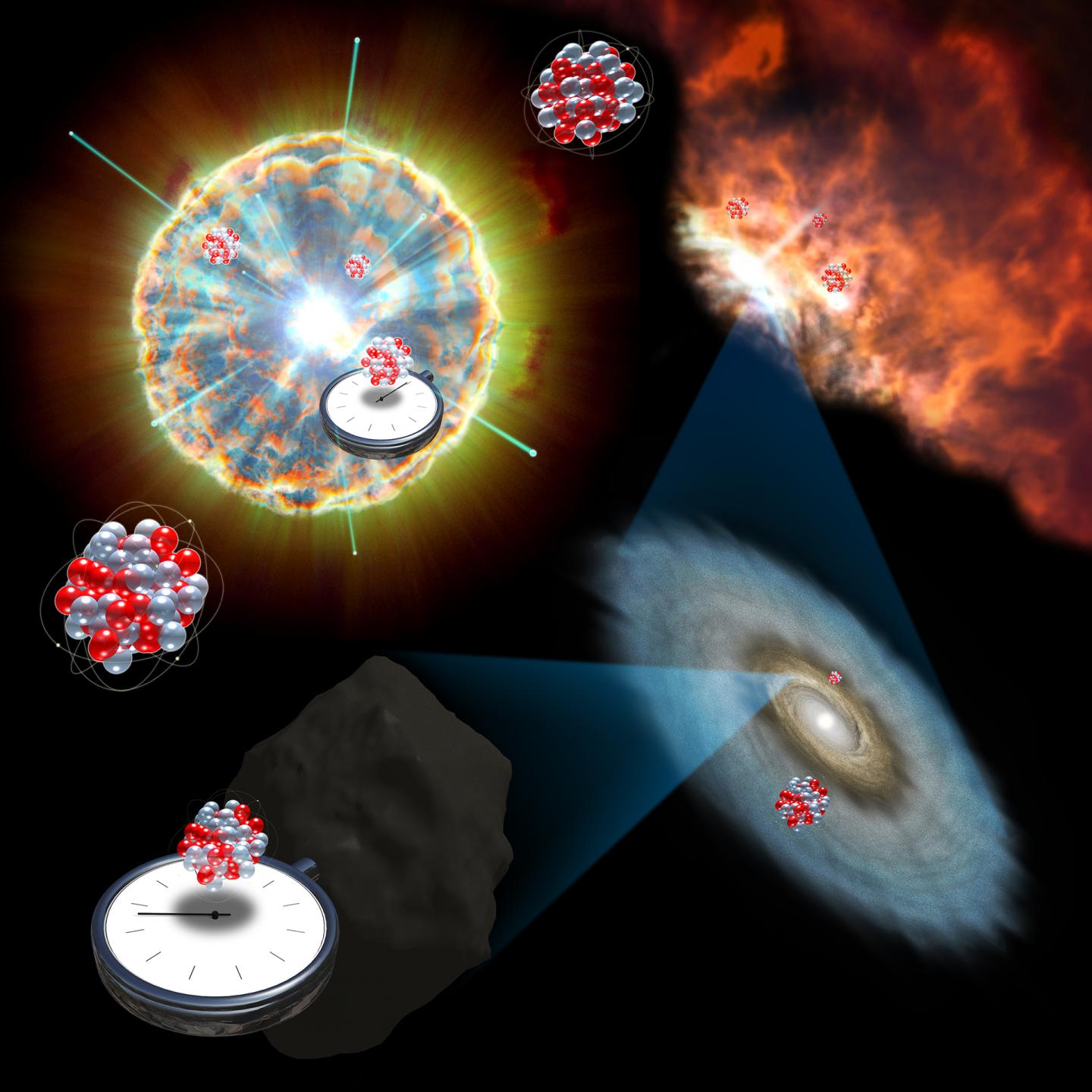
Billions of years ago, a huge star blasted open and spewed its guts into space. At that energetic moment, the so-called core-collapse supernova formed a debris cloud of brand-new atoms, forged in the heat of its blast. Time passed. The cloud contracted, attracted to itself by its own gravity. A star formed — our sun — surrounded by chunks of rock and gas that formed our planets and other orbiting bodies. Much later, we came along.
That's the basic story of our solar system's birth. And, mostly from watching other supernovas and other star births out in space, scientists know a fair amount about it. But there's still a lot about what happened during the stellar blast that's mysterious. What exotic, energetic particles flared into being in that first, hot flash of the old star's death? How did they shape the atoms and molecules that formed humans? How much time passed between the star's death and rebirth as our sun?
In a new paper published yesterday (Sept. 4) in the journal Physical Review Letters, researchers proposed a new method for answering those questions. [The Coolest Little Particles in Nature]
When the old star exploded, a rare sort of ghostly antimatter version of a neutrino particle — called the "electron anti-neutrino" — burst into being and slammed into the surrounding matter of the supernova. Those collisions helped to produce an isotope of the element technetium called 98Tc. And if researchers knew how much 98Tc was produced and what happened to it, they'd be able to describe that dying blast in much more detail. They'd also be able to calculate much more precisely how long ago that supernova happened.
But the thing about 98Tc is that it falls apart quickly after it's created, decaying into an isotope of the element ruthenium, called 98Ru. And there wasn't that much of it in the first place.
The researchers proposed in their paper, however, that traces of 98Tc might be relatively simple to detect and measure in meteors that sometimes fall to Earth, since those ancient rocks have largely been untouched since the birth of the solar system. And they calculated that the electron anti-neutrinos of our birth supernova should have produced just barely enough 98Tc that its decay products would be detectable in meteors all these billions of years later.
With patience and careful measurement, they wrote, experimentalists could precisely measure those traces. And with a precise enough measurement, they might unlock the secrets of that enormous explosion that makes up the ancient history of nearly every atom in your body.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Originally published on Live Science.

