Facts About Vanadium

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Vanadium is a medium-hard, steel-blue metal. Although a lesser-known metal, it is quite valuable in the manufacturing industry due to its malleable, ductile and corrosion-resistant qualities.
Vanadium rarely exists as a free element in nature but can be found in about 65 different minerals, including magnetite, vanadinite, carnotite and patronite. It also can be found in phosphate rock and some crude oils. Vanadium is usually obtained by heating crushed ore in the presence of carbon and chlorine to produce vanadium trichloride, which is then heated with magnesium in an argon atmosphere, according to Jefferson Lab. Around 98 percent of mined vanadium ore comes from South Africa, Russia, and China.
Vanadium makes up 150 parts per million (ppm) of the Earth's core and comprises 0.019 percent of the Earth's crust, according to PeriodicTable.com. The cosmic quantity of vanadium in the universe is about 0.0001 percent. Vanadium can be detected spectroscopically in the Sun's rays and occasionally in the light of other stars.
Just the facts
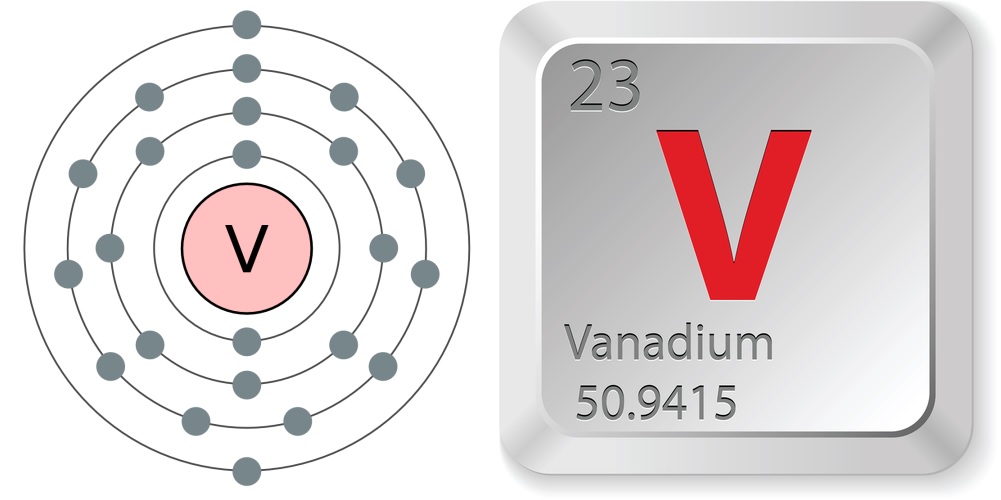
- Atomic number (number of protons in the nucleus): 23
- Atomic symbol (on the periodic table of the elements): V
- Atomic weight (average mass of the atom): 50.9415
- Density: grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 3,470 degrees Fahrenheit (1,910 degrees Celsius)
- Boiling point: 6,165 F (3,407 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 2; one stable V-51; one radioactive V-50
- Most common isotopes: V-51 (99.75 percent natural abundance)
Discovery
Vanadium was discovered twice, according to the Royal Society of Chemistry. In 1801, Andrés Manuel del Rio, a professor of mineralogy in Mexico City, discovered it in a specimen of vanadite. He sent samples of the element (which he had named erythronium) along with a letter describing his methods to the Institute de France for inspection and confirmation. Tragically, his letter was lost in a shipwreck, according to the Jefferson Lab, and the Institute received only his sample, along with a short note telling them how the new element resembled chromium. The Institute concluded that the sample was, in fact, a chromium mineral, and del Rio withdrew his claim.
Later, in 1830, vanadium was rediscovered by Swedish chemist Nils Gabriel Sefstrôm as he was analyzing samples of iron from a mine in Sweden. Possibly inspired by the element's beautifully-colored compounds, Sefström named the element vanadium after the Old Norse goddess Vanadis, the old Norse name for Freya, who is associated with beauty and fertility.
The element was finally isolated in 1867 by the English chemist Sir Henry Enfield Roscoe when he was combining vanadium trichloride (VCl3) with hydrogen gas (H2).
Trace mineral
High doses of vanadium are toxic to humans, but scientists think we may need the element in very small amounts for normal bone growth. Vanadium can be found in trace amounts in many types of food, including mushrooms, black pepper, parsley, dill weed, shellfish, beer, wine and grain. When we eat a balanced diet, we consume just 0.01 milligrams per day, and this is more than enough for our biological needs, according to the Royal Society of Chemistry.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Very few vanadium studies have involved humans (most have been conducted on animals), so vanadium isn't currently recommended for any particular diseases or health conditions. However, several animal studies and a few small human studies suggest that vanadium may lower blood sugar levels and improve insulin sensitivity in patients with type 2 diabetes, according to the University of Maryland Medical Center.
In some studies on mice, for example, vanadium was shown to lower blood sugar and levels of low-density lipoprotein (LDL) cholesterol and triglyceride. In a 1994 study on goats, published in the Journal of the American Dietetic Association, goat kids whose mothers consumed a diet deficient in vanadium were born with skeletal deficiencies and died three days later.
Many of the human studies with vanadium involved small numbers of people for short periods of time and with high doses, well above the tolerable upper intake level (UL), and scientists don't know if these levels are even safe for human consumption.
Vanadium is sold as a bodybuilding supplement typically in the form of vanadyl sulfate. The supplement is marketed as a way to increase muscle uptake of glucose and amino acids and enhance the synthesis of glycogen and protein. However, according to some scientific research, vanadium does not appear to enhance insulin activity in healthy people, only in diabetics, according to Encyclopedia.com.
Uses
Around 80 percent of the vanadium produced is alloyed with iron to make a shock- and corrosion-resistant steel additive called ferrovanadium, according to Jefferson Lab. Ferrovanadium contains between 1 to 6 percent vanadium.
Vanadium-steel alloys are used to make extremely tough tools such as axles, armor plates, car gears, springs, cutting tools, piston rods and crankshafts. Vanadium alloys are also used to make nuclear reactors because of their low-neutron-absorbing properties, according to the Royal Society of Chemistry. In fact, the first widespread industrial use for vanadium was in the steel framework of the Model T Ford, which allowed for a lighter weight frame that was also of greater tensile strength.
The compound vanadium pentoxide (V2O5) is used as a mordant (a substance that permanently fixes dyes to fabrics), as a catalyst in some chemical reactions and in the manufacturing of ceramics. It can also be combined with gallium to form superconductive magnets, according to Jefferson Lab. When mixed with aluminum and titanium, vanadium can create a very strong alloy that is used for special applications such as dental implants and jet engines.
Who knew?
- Inhaling large amounts of vanadium can result in lung problems, such as bronchitis or pneumonia. It has been shown that workers exposed to vanadium peroxide are more susceptible to eye, nose and throat irritation.
- Vanadium was used to make portable artillery pieces and body armor in World War I.
- In a 1996 study published in the medical journal Metabolism, eight people with type 2 diabetes were given vanadium supplements for one month. It appeared moderately successful in lowering blood sugar levels with few side effects. During the first week, six of the eight participants experienced some gastrointestinal problems, but these side effects disappeared with continued use.
- Vanadium has very colorful oxidation states, including purple, green, blue and yellow.
- In 1911, German chemist Martin Henze discovered vanadium in the blood cells of sea squirts.
- Vanadium atoms have 23 electrons, 28 neutrons and 23 protons.
- Since vanadium steel keeps its hardness at high temperatures, it is used in circular saws, drill bits, engine turbines and other moving parts that produce high levels of heat.
- Vanadium can be used in the process of refining uranium for nuclear purposes.
- Vanadium is present in a small number of meteorites.
Additional resources

 Live Science Plus
Live Science Plus