Facts About Gallium

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Gallium is a soft, silvery metal used primarily in electronic circuits, semiconductors and light-emitting diodes (LEDs). It is also useful in high-temperature thermometers, barometers, pharmaceuticals and nuclear medicine tests. The element has no known biological value.
Natural element
In nature, gallium is never found as a free element and cannot be found in a substantial amount in any minerals. Rather, it exists in trace amounts in various compounds, including zinc ores and bauxite. By weight, gallium makes up about 0.0019 percent of Earth's crust, according to PeriodicTable.com. It is easily obtained by smelting, however, and most commercial gallium is extracted as a byproduct of aluminum and zinc production, according to Chemicool. The largest producers of gallium are Australia, Russia, France, and Germany.
Just the facts
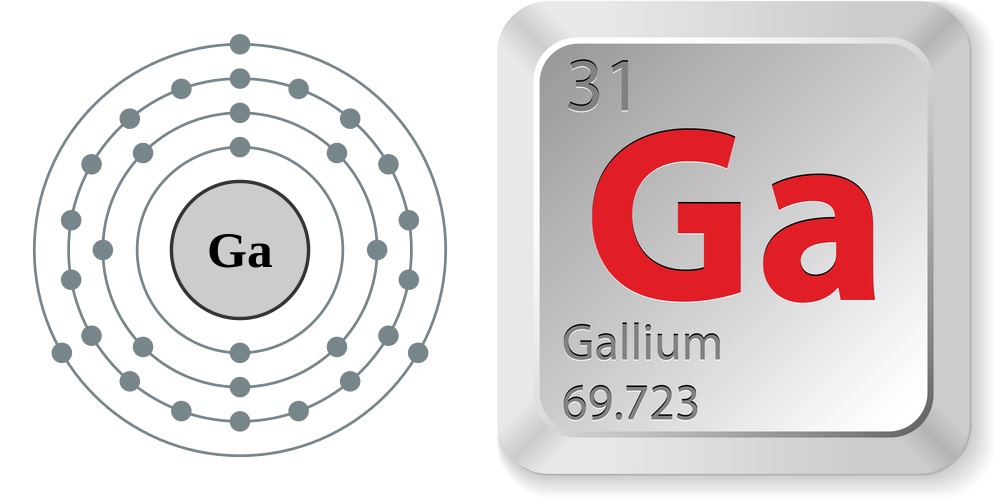
- Atomic number (number of protons in the nucleus): 31
- Atomic symbol (on the periodic table of the elements): Ga
- Atomic weight (average mass of the atom): 69.723
- Density: 5.91 grams per cubic centimeter
- Phase at room temperature: solid
- Melting point: degrees 85.57 degrees Fahrenheit (29.76 degrees Celsius)
- Boiling point: 3,999 F (2,204 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 24 whose half-lives are known
- Most common isotopes: Two stable Ga-69 (natural abundance 60.1 percent) and Ga-71 (natural abundance 39.9 percent).
A unique metal
On the periodic table of the elements, gallium is grouped in the Boron family (group 13), which includes the semi-metal boron (B) and the metals aluminium (Al), gallium, indium (In) and thallium (Tl), according to Chemistry LibreTexts. All five of these elements have three electrons in their outer energy level.
Gallium is a post-transition metal. These are metallic elements located between the transition metals and the metalloids (non-metals) on the periodic table. Post-transition metals have some of the traits of the transition metals but tend to be softer and conduct more poorly. The post-transition metals include some of the Boron family elements — aluminum, indium and thallium — but also tin (Sn), lead (Pb) and bismuth (Bi).
Gallium has some very unique qualities. For example, although it is a solid at room temperature (about 77 F/ 22 C), it is still so soft that you could cut it with a knife. In addition, it has a low melting point of 85.57 F (29.76 C) — less than 10 degrees above room temperature — so if you were to pick up a lump of gallium, it would literally melt from the warmth of your hand. Then if you set it back down, it would solidify again.
Even with such a low melting point, gallium's boiling point is quite high at 3,999 F (2,204 C), giving it one of the greatest ratios between melting point and boiling point of any element. At low temperatures, gallium is a brittle solid that breaks quite easily, and similar to glass, it shatters conchoidally (does not follow natural planes of separation).
Get the world’s most fascinating discoveries delivered straight to your inbox.
Uses
Gallium is used primarily in electronics. In fact, about 95 percent of all gallium produced is used to make gallium arsenide (GaAs), a compound used in microwave and infrared circuits, semiconductors and blue and violet LEDs, according to Chemistry Explained. Gallium arsenide can produce laser light directly from electricity and is used in solar panels, including those on the Mars Exploration Rover. The compound gallium nitride (GaN) is used as a semiconductor in Blu-ray technology, mobile phones and pressure sensors for touch switches.
Gallium bonds easily with most metals and is commonly used to make low-melting alloys. It is one of four metals (including mercury, rubidium and caesium) that are liquid at or near room temperature. Of these four metals, gallium is the least reactive and least toxic, making it the most safe and environmentally-friendly choice for high temperature thermometers, barometers, heat transfer systems and cooling and heating devices.
Liquid gallium can be quite difficult to work with, however, as it clings to glass, skin and most other materials (except graphite, quartz and Teflon). It also expands when it freezes so it cannot be stored in glass containers.
Gallium is also used in some pharmaceuticals and radiopharmaceuticals. For example, the radioactive isotope Ga-67 is used as a nuclear medicine test to look for inflammation, infection or cancer in the body.
Gallium nitrate is used in many pharmaceuticals and as a treatment for hypercalcemia, a disease which can lead to the growth of bone tumors. Gallium has also been suggested as a treatment for cancer, infectious disease and inflammatory disease. However, exposure to large amounts of gallium may cause throat or chest irritation, and the fumes can lead to some serious conditions, according to Chemistry LibreTexts.
Discovery
Before gallium was discovered, it was predicted by Russian chemist and inventor Dimitri Mendeleev, the creator of the periodic table of elements. He named the missing element eka-aluminum because he knew it would go below aluminum on the periodic table in box 31, according to Chemicool.
The element was first discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, who had been studying the spectra of the chemical elements for 15 years (spectra are the lines produced when chemical elements are heated), according to Chemistry Explained. Since each element produces its own distinctive set of lines, or spectra, this method was a reliable way to identify elements.
Lecoq de Boisbaudran wondered if element 31 might be found in zinc ores. Zinc, which has an atomic number of 30, sits next to gallium on the periodic table. In August 1875, using a spectroscope, Lecoq de Boisbaudran did indeed find some gallium, but only in very small amounts. He reported that the spectrum of the new element was composed of a narrow, readily visible, violet ray, according to Chemistry Explained.
Later that year, Lecoq de Boisbaudran obtained pure gallium through the electrolysis of gallium hydroxide in potassium hydroxide, according to the Jefferson Lab. Lecoq de Boisbaudran was then given several tons of zinc ore by miners for his research. From this ore, he was able to produce a few grams of nearly pure gallium, according to Chemistry Explained. Lecoq de Boisbaudran proposed the name gallium for the new element, which comes from the Latin word "Gallia," meaning France.
Who knew?
- Liquid gallium cannot be kept in a glass or metal container because it expands 3.1 percent when it freezes.
- Gallium has the largest liquid range of any metal.
- The Neutrino Observatory in Italy uses large amounts of gallium trichloride to study solar neutrinos produced in the sun. Neutrinos are particles created in the first second of the universe — even before atoms — and are continually being made through nuclear reactions of the sun and other stars, according to the University of Wisconsin-Madison.
- In its purest extracted form, gallium is a vibrant silver color. As a solid, it is blue-gray.
- Another unusual trait of gallium is that it can be supercooled quite easily. Supercooling is the cooling down of a substance below its freezing point without turning it into a solid.
- Approximately 95 percent of gallium consumption is in the electronics industry, according to LibreTexts. Gallium arsenide and gallium nitride represent about 98% of gallium consumption in the United States.
- Gallium has been used in nuclear bombs to stabilize the crystal structure.
- When painted on glass, gallium turns into a lustrous mirror.
- Gallium's boiling point is more than eight times higher than its melting point on the absolute scale — the greatest ratio between melting point and boiling point of any element.
Additional resources

 Live Science Plus
Live Science Plus