
One molecule could usher revolutionary medicines for cancer, diabetes and genetic disease — but the US is turning its back on it
The U.S. government is divesting from mRNA vaccines, but will other uses of the technology be spared? In a time of uncertainty, scientists worry that revolutionary treatments for cancer, immune dysfunction and genetic disease may be left on the lab bench.

On Dec. 31, 2019, the first reports emerged about a mysterious pneumonia of unknown cause circulating in China. On March 11, 2020, the World Health Organization declared COVID-19 a pandemic.
On March 16, the first COVID-19 vaccine entered clinical trials.
And by Dec. 14, members of the American public were getting the first doses of COVID-19 vaccines outside of trials.
The first coronavirus vaccines were paradigm-shifting because they went from conceptualization to mass production in mere months. But they were also unique because they used a new way to stimulate the immune system — one that had been thoroughly studied for decades in order to be ready for deployment at this crucial moment.
The key to these vaccines was messenger RNA (mRNA), DNA's less-famous cousin. The power of the mRNA platform is that vaccines can be produced exceptionally quickly once a pathogen's genetics have been analyzed; conventional vaccine manufacturing takes months or years whereas mRNA vaccines can be made in mere weeks. So while it was once the subject of high school biology classes and niche pockets of biomedical science, mRNA was suddenly thrust into the public eye — and once there, it inspired relentless misinformation and controversy.
While mRNA-based COVID-19 vaccines are the best known application of the molecule, researchers around the world have found additional uses for mRNA technology beyond the realm of vaccines. They're exploring its use for groundbreaking treatments for cancer and autoimmune disease, as well as for gene-editing therapies for genetic disorders. But that promise may be unrealized in the United States, where the federal government has declared war against this promising technology.
This new stance runs counter to the Trump administration's prior embrace of mRNA vaccines.
Get the world’s most fascinating discoveries delivered straight to your inbox.

"We do really have to give President Trump credit for introducing the mRNA platform to the world through his leadership in Operation Warp Speed," said Jeff Coller, the Bloomberg distinguished professor of RNA biology and therapeutics at Johns Hopkins University. "The president should be taking a victory lap." But instead, the second Trump administration is actively dismantling this legacy, Coller told Live Science.
Vaccine skeptic Robert F. Kennedy Jr. now heads the Department of Health and Human Services (HHS), and opponents of both conventional and mRNA-based vaccines hold seats on the country's most influential vaccine advisory committee. Since Trump's inauguration, federal scientists have faced mass layoffs, funding freezes, and memos warning them to disclose their involvement in research areas the administration has targeted, including mRNA vaccines.
These actions had an immediate chilling effect on mRNA research and development in the U.S., Coller told Live Science. And then, in August, HHS canceled nearly half a billion dollars of investment into mRNA vaccine development.
"I was shocked to see this, frankly," said Jordan Green, head of the Biomaterials and Drug Delivery Laboratory at Johns Hopkins, whose lab is developing both mRNA therapies and delivery systems to get the molecule into the body.
The cuts are sending ripples through biotech, making stakeholders question whether it's safe to set up shop in the U.S., or whether their mRNA investments would be better spent abroad. "It's just a shame because it's an unforced error; there's no reason," Green said.
For now, HHS appears to be retreating primarily from mRNA vaccines; it noted "other uses of mRNA technology" would not be affected by the cuts. But "the industry doesn't trust that," Coller told Live Science.
According to Grant Witness, a project tracking scientific grants under the Trump administration, mRNA research unrelated to vaccines has already been hit by grant terminations and funding freezes. So even if it's not being explicitly targeted, it's not necessarily being preserved. The project's database shows that the National Institutes of Health (NIH), part of HHS, terminated grants for projects developing mRNA-based treatments for cancer, Alzheimer's, pulmonary arterial hypertension and HIV, as well as grants for basic research about how mRNA works in healthy and diseased cells.
Here's what the United States stands to lose if the federal government broadly divests of mRNA medicines after spending decades readying the technology for prime time.
Cancer treatment
On a molecular level, mRNA is a close cousin of DNA — and human cells are stuffed with it. These ubiquitous "messengers" copy instructions from DNA and relay them to other locations in the cell — namely, to protein-construction sites where the complex molecules that do most of the work in cells get made. mRNA also performs other key jobs in cells, such as helping control which genes are switched on and to what degree.
For decades prior to the pandemic, dedicated scientists pored over mRNA, learning how this class of molecules works in the body and how it might be leveraged to heal the sick and guard against disease.
"I started doing this when I was like 21, back in the '90s, back when about 10 people on Earth knew what mRNA were," Coller said. Findings from Coller's lab later helped inform the development of Spikevax, the COVID-19 vaccine made by Moderna. But while COVID-19 vaccines are the best-known application of mRNA to date, they're far from the first.
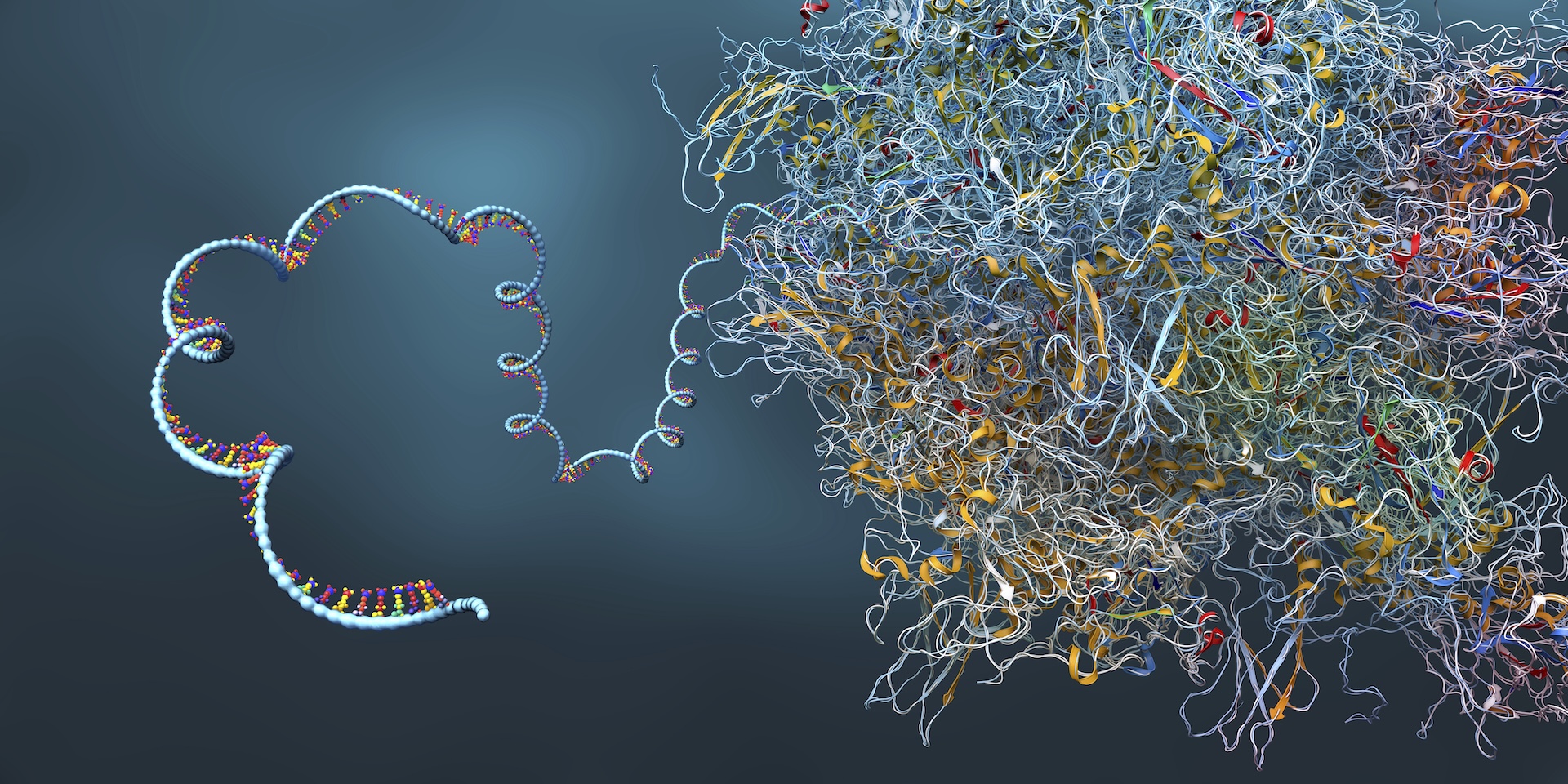
The first mRNA therapeutics company was founded in 1997, and rather than targeting infectious disease, it had its sights set on cancer treatment. Its approach ultimately hasn't panned out in human trials, but in the meantime, other approaches to mRNA-based cancer treatment have gained traction.
Cancer vaccines are a standout example, but in this context, the term "vaccine" is "a bit of a misnomer," said Dr. Vinod Balachandran, a pancreatic cancer surgeon-scientist and director of The Olayan Center for Cancer Vaccines (OCCV) at Memorial Sloan Kettering Cancer Center. Rather than being given preventatively, like a COVID vaccine, cancer vaccines are "given to patients as a treatment; it is a therapy," he said.
These therapies are similar to conventional vaccines in that they train the immune system to recognize antigens, which are substances that act as "red flags" for foreign invaders, toxins or diseased cells. These include, for example, the spike protein of SARS-CoV-2 — the virus that causes COVID-19 — and certain molecules on cancer cells. Balachandran and colleagues have focused on pancreatic cancer, which has a five-year relative survival rate of only 13% — meaning people newly diagnosed with pancreatic cancer are about 13% as likely to survive the next five years compared with the general population.
The researchers have found that, in the rare long-term survivors of pancreatic cancer, the immune system can recognize the cancer and fight off its recurrence. The team hoped to re-create that immune recognition in other patients by analyzing the genetics of their tumors to see what unique antigens they express. They then create customized vaccines that target those molecules.
"We felt at the time [when we started this work] — this is back in 2017 — that the best technology for rapid custom cancer vaccination for patients was to use RNA," Balachandran said. Once you know a given patient's cancer genetics, a personalized mRNA vaccine that targets multiple antigens can be crafted in a matter of weeks. Conventional vaccines that require the antigens to be grown in the lab and purified would take many months to make.
"For cancer vaccination, speed is essential," Balachandran said. "These are patients who are facing deadly cancers, who require rapid treatment. So we do not have the luxury of waiting."
So far, the team has seen some success. In an early-stage trial, they treated 16 patients who had undergone surgery for pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer, which has a five-year survival rate of about 10% to 12%, though they fare a bit better when their cancer can be surgically removed.
Within nine weeks of surgery, Balachandran said, each patient's tumor tissue was analyzed and a personalized vaccine was crafted and delivered alongside post-surgery cancer treatments, like chemotherapy. Half of the patients responded to their vaccine, producing immune cells that have persisted for nearly four years so far, and estimates suggest they could last an average of seven years, "with some lasting even beyond a decade," Balachandran said.
The vaccine responders had a significantly lower risk of recurrence in the following three years than those who didn't respond, with six showing no signs of recurrence in that time frame. A mid-stage trial is now testing the vaccine in about 260 people to see how well it delays or prevents recurrence compared with standard treatment.
"Anything we can do to improve outcomes for these patients who really need help I think will be transformative for the field, for them, for their families — it will mean a lot," Balachandran said. "All other immune therapies and all other therapies have largely failed."
In this initial trial, vaccine production took over two months, in part because samples had to be shipped to overseas collaborators at BioNTech. "We are confident this can happen much faster" in the future, perhaps within a month, Balachandran said.
Other scientists are working on off-the-shelf cancer vaccines that can help bridge the gap until a patient gets a personalized one. These vaccines use a mix of mRNA molecules to stir up a generic, first-line immune defense against cancer. In mice with different kinds of solid tumors, researchers led by Dr. Elias Sayour, a pediatric oncologist at the University of Florida, demonstrated that such a vaccine triggers anti-cancer responses by itself. And when used in combination with another cancer treatment, the mRNA mix can boost the effects of that therapy, Sayour's team has found.

That team has now moved on to human patients, with a two-pronged approach: an off-the-shelf cancer vaccine, followed by a personalized one. They have also run trials of personalized vaccines for the deadly brain cancer glioblastoma, finding that the shots mount a strong, targeted immune response against these tumors that are usually difficult for the immune system to "see." Meanwhile, at another lab, scientists are testing a personalized vaccine in late-stage trials for the skin cancer melanoma and non-small cell lung cancers.
Pancreatic cancer has historically been an elusive target for the immune system because, compared to cancers like melanoma, its cells carry relatively few antigens, Balachandran explained. But pancreatic cancer can be targeted with these vaccines. That bodes well for using mRNA approaches to treat potentially many other cancers, and "I think that's really the most exciting take-home," he said.
Beyond cancer vaccines, Green and colleagues are leveraging mRNA to fight the disease in a different way: by forcing tumors to raise their own red flags to the immune system. Using mRNA packaged inside nanoparticles, the researchers introduce immune-cell genes into cancer cells, prompting tumors to expose their antigens and secrete molecules that call immune cells to the area.
"We can program that tumor cell to now act like an immune cell that helps teach other immune cells what its antigens look like, how to recognize it, how to destroy it," Green told Live Science. In mouse models of breast cancer and melanoma, they combined this approach with an existing immunotherapy and found that it helped shrink and clear tumors from the body while also extending survival.
If shown to work in people, "this could just be off the shelf," he said. It would be one injectable that could work on any patient's solid tumor, he said.
Immune reprogramming
In cancer, scientists are exploring different ways to launch an immune attack against tumor cells, whether by igniting a generalized response or enabling cells to spot specific red flags. But in some diseases, the immune system itself is the culprit — and in those instances, mRNA can help rein in turncoat immune cells.
"Autoimmune diseases — type 1 diabetes, multiple sclerosis, Crohn's, colitis — these can be treated with these mRNA and genetic-based therapies," potentially, Green said. The goal of these treatments would be to "tune the immune system" so it stops attacking healthy tissues.
In type 1 diabetes, for example, the immune system attacks beta cells, which make insulin, leaving the body too little of the hormone to control blood sugar. Green and colleagues are in the early stages of developing an mRNA medicine to reprogram the immune system so it better tolerates beta cells, rather than attacking them. They aim to do this by targeting special immune cells in the liver that promote a tolerant environment in the organ.
The liver is constantly exposed to antigens from food and from microbes in the gut, so immune activity is dialed down to prevent an overreaction. The team's idea is to deliver mRNA that codes for beta-cell proteins to the liver, essentially marking those beta-cell proteins as "safe" to the immune system. This, in turn, can increase the number of regulatory T cells that recognize the proteins as safe; regulatory T cells keep other immune cells in check, and could thus help ward off further attacks on beta cells.
"The problem with autoimmunity is that [the immune system] thinks parts of its own body are foreign," so it's trying to attack them, Green explained. The hope is that "we can use these mRNA medicines to train the immune system so that it sees, 'Oh no, this is fine.'" The work is currently in preclinical stages, as the team runs experiments with cells and lab mice to refine their mRNA nanoparticles.
Green calls this approach of fine-tuning specific cells' activity "genetic surgery." Rather than using a "blunter instrument," like immunosuppressive drugs, to broadly suppress the immune system, the surgery makes a precise change to counter only harmful immune activity. In the long run, the lab is looking to apply this same approach to other autoimmune diseases beyond diabetes, such as multiple sclerosis, in which immune cells target myelin, the insulation surrounding nerve fibers in the brain and spinal cord.
Meanwhile, BioNTech has published data from its own early tests of an mRNA vaccine designed to quell autoimmunity against myelin. In mice, the vaccine expanded populations of immune cells that then kept the myelin-attacking cells in check, suppressing their activity without hobbling the immune system as a whole.
Elsewhere, researchers including Dr. Samira Kiani of the University of Pittsburgh School of Medicine and Kathryn Whitehead of Carnegie Mellon University are using mRNA to counter off-the-rails immune responses, such as the extreme inflammation seen in sepsis. Their approach, described in a preprint, packages mRNA inside a tiny bubble of fat called a lipid nanoparticle (LNP), which then ferries the mRNA into immune cells. From there, the mRNA instructs the cells to make proteins called "zinc finger repressors," which latch onto genes and suppress their activity. In this case, they repressed a key gene involved in the immune signaling that can lead to runaway inflammation.
In experiments with cells in lab dishes and with mice, this type of "epigenetic engineering" showed promise as a potential method for tuning the activity of the immune system. One perk of mRNA is that it degrades quickly in the body, the study authors wrote, so in theory, once the harmful inflammation is subdued, the immune system can get back to defending the body against germs.
Gene editing
mRNA is also being used in tandem with the gene-editing system CRISPR to revolutionize treatments for genetic disease.
Classical CRISPR systems use molecular scissors to snip through DNA strands and enable scientists to tweak specific segments of the molecule. And now, a modified system called base editing can be used to precisely change just one "letter" in DNA's code.
But if you want to use CRISPR for gene therapy, you must first get the scissors, as well as a "guide" molecule that directs them to the right spot — into human cells. And, ideally, the scissors enter only the cells you need to edit, said Giedrius Gasiūnas, a senior researcher in the Life Sciences Center at Vilnius University in Lithuania.
That's where mRNA packaged in nanoparticles could be a game changer.
"This technology could be important for in vivo delivery," meaning the delivery of gene editors directly into the body, said Gasiūnas, who is also chief scientific officer of the biotech company Caszyme.
The first CRISPR-based therapy approved in the U.S. involved editing cells outside the body. The therapy treats two blood disorders by disabling a gene called BCL11A; patients have blood-making stem cells removed from their bone marrow, edited in the lab and then returned to their bodies. But this complex treatment involves a month-long hospital stay, during which the edited stem cells give rise to new blood cells. By comparison, mRNA approaches to gene therapy would be easier to administer and thus more likely to be scalable, Gasiūnas said.
mRNA isn't the only vehicle for getting CRISPR therapies into the body. Some existing gene-editing treatments instead use harmless viruses, such as adeno-associated virus (AAV) vectors, and this same approach is being applied to CRISPR in some emerging therapies. But mRNA packaged inside nanoparticles is emerging as a key player in the field.
This application made headlines in the case of KJ, an infant who became the first person to receive a personalized CRISPR treatment. "The CRISPR technology was introduced as an mRNA," Coller said. "That is the critical feature that was necessary to get this to work."
"It's foolish to condemn mRNA because people didn't like vaccine mandates or mask mandates or whatever they didn't like."
Dr. Seth Berkley, Brown University School of Public Health
KJ was born with a severe form of carbamoyl phosphate synthetase 1 (CPS1) deficiency, an inherited disease that causes ammonia to accumulate in the body and affects an estimated 1 in 1.3 million people worldwide. The condition arises from mutations in the CPS1 gene, but in different patients, the gene is broken in different ways. To fix KJ's specific mutation, scientists developed a customized gene therapy consisting of a guide and an mRNA that carried instructions for a base editor into his liver cells, where CPS1 is most active.
"The editor is so large that it cannot easily be accommodated by an AAV vector," said Dr. Kiran Musunuru, co-developer of the therapy and a professor at the University of Pennsylvania's Perelman School of Medicine. Because other potential delivery systems are too early in development, "LNPs are currently the best option," Musunuru told Live Science in an email.
The therapy was ready to administer within six months of KJ's birth. By 9 months old, he had received several doses of the therapy and was thriving; he was hitting milestones he may have never reached otherwise, his medical team reported in May. Had he not received the treatment, he would have needed a liver transplant once he got big enough.
Promising technology at risk
When it comes to the future of mRNA medicines in the U.S., vaccines for infectious disease currently have the bleakest outlook due to political barriers being raised around the technology. Among mRNA tech, vaccines have been the primary target of the federal government's ire, despite experts' warnings that abandoning the shots will leave us vulnerable to pandemics.
"It's foolish to condemn mRNA because people didn't like vaccine mandates or mask mandates or whatever they didn't like," Dr. Seth Berkley, an epidemiologist and senior adviser to the pandemic center at the Brown University School of Public Health, told Live Science. "What we need to do is improve upon them [mRNA vaccines] and understand what the best use of them is," which can only be accomplished through investing in further research.

For now, other applications of mRNA don't appear to be a focus for funding cuts, although some projects have nonetheless been caught in the crosshairs of reduced federal spending. For his part, Musunuru told Live Science he's been in discussions with the Food and Drug Administration regarding ways to get more patients access to personalized gene therapies like KJ's, so that the technology isn't used only in a handful of special cases. When asked about potential funding cuts, he added, "Vaccines and therapies are so different that I do not expect the latter to be affected."
Green, however, anticipates that divestments from vaccines would have unanticipated impacts across the field, because mRNA therapies and vaccines are ultimately composed of very similar genetic molecules and delivery systems.
Regarding nanoparticles, "the vaccine development is … at the pioneering edge of this technology platform," Green said. He argues that cuts to vaccine R&D will inevitably undermine the development of nanoparticles that could be really useful in treating cancer or genetic disease. "It's going to set us back in a very broad way," he said.
The hits to vaccine research also aren't happening in isolation. Myriad federal science grants have been terminated or frozen; NIH agencies may be facing steep funding cuts; and the government shutdown ushered mass layoffs across leading health institutions. Sayour, the oncologist whose lab is developing personalized and universal cancer vaccines using mRNA, said federal grants have historically been critical to his research.
"None of this would be possible without the support of the federal government," Sayour told Live Science. "That is most certainly, 100% true." And that goes for every stage of research, from studies in lab dishes to large clinical trials.
These widespread cuts and more-targeted cuts to mRNA vaccines will not only starve the academic environments where new biotechnologies are typically nurtured, Coller said; they will also drive prospective talent, investors and industry stakeholders in the mRNA space away from the U.S. "In biotech, we're going to see, over the next five to 10 years, a significant 'brain drain,' where other countries build up their infrastructure," he said.
Coller knows this because he is a founding member of the Alliance for mRNA Medicines (AMM), a global organization aimed at advancing and advocating for mRNA medicines. In the spring, AMM surveyed over 100 leaders in the field. They primarily represented U.S.-headquartered pharmaceutical and biotechnology organizations but also included some based in Europe, Canada or Asia. Even prior to the major HHS cuts in August, these stakeholders were feeling squeezed; nearly half reported "already experiencing direct impacts" from federal policy changes tied to mRNA funding.
"Let's not fool ourselves: mRNA is one of the three most important molecules in the body, with the other two being DNA and protein. It's the intermediary between them."
Jeff Coller, Johns Hopkins University
The cuts caused projects to be scaled back, partnerships to be terminated, and jobs to be lost. About 30% of respondents said that, if faced with further cuts, they might pivot away from mRNA, and 30% said they'd consider moving their operations to other countries. Over 80% agreed that anti-mRNA policies would drive talent away from the U.S. and toward other locales.
Even as scientists contend with these big-picture problems on the national and international stage, skirmishes have also been unfolding at the state level. These battles primarily concern public access to mRNA vaccines but could potentially have broader ripple effects.
A bill still in committee in Iowa would make administering an mRNA vaccine in the state a misdemeanor with a fine of $500 for each shot given. Another bill in committee in South Carolina would prohibit the use of mRNA-based "gene therapies" in the state, but makes an exception for therapies for noninfectious diseases, such as cancer.
It may be that most bills of this ilk never become law and some, like the one raised in South Carolina and two dead bills in Texas, purposely make exceptions for certain uses of mRNA. However, others have been worded in ways that made it unclear whether any mRNA medicine is permissible. If any of such bills ultimately pass at the state level, that could sway where mRNA companies can manufacture products and run clinical trials, Coller said. So, while an exodus of expertise unfolds at a national level, we may see an echo of that at the state level.
The future of mRNA medicine in the U.S. rests on many "ifs," and the worst-case scenario may not come to pass. But in the face of staggering uncertainty, researchers in the field are starting to look for better bets.
"Let's not fool ourselves: mRNA is one of the three most important molecules in the body, with the other two being DNA and protein. It's the intermediary between them," Coller said. "When the federal government sends a message that mRNA-based medicine and research is not wanted, you're basically saying that there's a whole branch of science that is no longer welcome within the U.S."

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus





