Antibiotic found hiding in plain sight could treat dangerous infections, early study finds
Scientists have discovered a never-before-seen antibiotic that shows promising in treating drug-resistant infections.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Scientists have reported the discovery of the first in a promising new class of antibiotics — and the find was unexpected, as they hadn't set out to find new drugs.
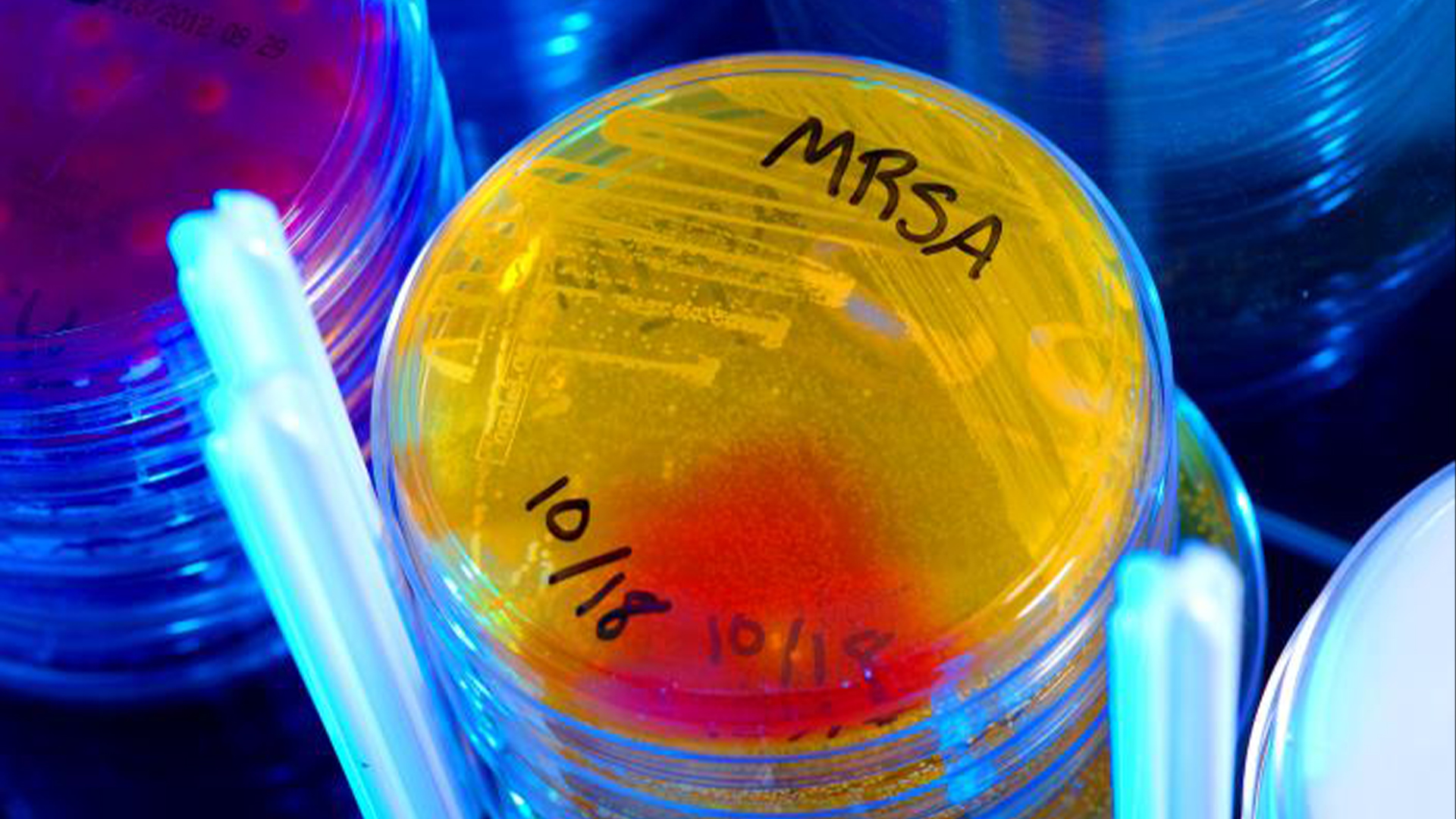
The new antibiotic compound shows promising activity against drug-resistant infections, including methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcus faecium, germs that are notorious for causing resistant infections in hospitalized patients.
The discovery of the novel molecule, called pre-methylenomycin C lactone, was reported Oct. 27 the Journal of the American Chemical Society and "can be considered the first in a new class" of antibiotics, Lona Alkhalaf and Greg Challis, chemists and lead authors of the study, told Live Science in an email.
However, the team's initial aim wasn't to discover new drugs. Instead,the researchers were investigating how a known antibiotic, methylenomycin A, gets made by a soil-dwelling bacterium called Streptomyces coelicolor.
Plants and microorganisms produce a host of complex compounds known as secondary metabolites, many of which happen to have useful medicinal properties in humans. Understanding how these compounds are made within these organisms and how they interact with human cells can help scientists develop effective drugs from these natural products.
The blueprints for producing these different biological molecules are held in specific collections of genes, called "biosynthetic gene clusters." By deleting individual genes from these clusters, Alkhalaf and Challis could remove specific enzymes involved in the synthesis of methylenomycin A. This research technique enabled them to halt the reaction sequence at key points to study it more closely — and it led them to spot extract previously unobserved intermediate compounds that get produced along the way.
This systematic approach allowed the team to isolate two never-before-seen molecules, which they named pre-methylenomycin C and pre-methylenomycin C lactone. After using an array of techniques to thoroughly characterize the structures of these compounds, they investigated the molecules' biological activity against a panel of bacterial strains.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Pre-methylenomycin C lactone proved particularly promising. "[It] is active against a range of Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and a multidrug-resistant strain of Enterococcus faecium," Challis and Alkhalaf told Live Science. "[It] is 100x better at killing drug-resistant bacteria than the original antibiotic."
But perhaps more importantly, the new compound doesn’t appear to induce antibiotic resistance in the treated strains.
Repeated exposure to antibiotics can trigger the evolution of defense mechanisms in some bacteria, eventually leading to drug resistance that makes future infections extremely difficult to treat. In a 28-day experiment, E. faecium bacteria were exposed to increasing concentrations of pre-methylenomycin C lactone, providing the perfect opportunity for resistance to develop. But over that period, the team did not observe any change in the minimum inhibitory concentration — the amount of drug needed to produce a visible effect. In short, the antibiotic retained its infection-killing activity, and the bacteria did not evolve any problematic resistance mechanisms.
Expanding this line of research — both to include more bacterial strains and to study the drugs' effects over a longer time frame — will be two important next steps for the team to demonstrate the full potential of the new molecule.
"It's a really nice study and I think this is a lesson: if you isolate novel molecules then look for novel activities with those molecules," said Stephen Cochrane, a medicinal chemist at Queen's University Belfast in Ireland who was not involved with the work. However, he cautioned that there is a significant difference between a compound with antibacterial activity and an antibiotic used to treat disease.
"The big challenge is translating this into a viable drug — something that persists long enough in the body, isn't toxic to humans, and is not prone to resistance," he said.
For Alkhalaf and Challis, this is exactly the direction they hope to pursue next. They are now collaborating with David Lupton, a synthetic chemist at Monash University in Australia, to develop a chemical route to pre-methylenomycin C lactone. This would enable them to produce the molecule from scratch via chemical synthesis, rather than relying on microbes to make it. In turn, this would provide larger volumes of the compound for studies aimed at understanding how the molecule works and how it might affect human cells.
"It would be useful to identify the biological target(s) of the compound in susceptible bacteria and develop a better understanding of how changes to the compound's structure affect target binding and biological activity," Alkhalaf and Challis said. Such insight might inform the design of related compounds with even more potent antibiotic activity.

Victoria Atkinson is a freelance science journalist, specializing in chemistry and its interface with the natural and human-made worlds. Currently based in York (UK), she formerly worked as a science content developer at the University of Oxford, and later as a member of the Chemistry World editorial team. Since becoming a freelancer, Victoria has expanded her focus to explore topics from across the sciences and has also worked with Chemistry Review, Neon Squid Publishing and the Open University, amongst others. She has a DPhil in organic chemistry from the University of Oxford.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus