Superbugs are on the rise. How can we prevent antibiotics from becoming obsolete?
Improving our "stewardship" of antibiotics is key to preventing bacteria from gaining resistance to the essential drugs.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
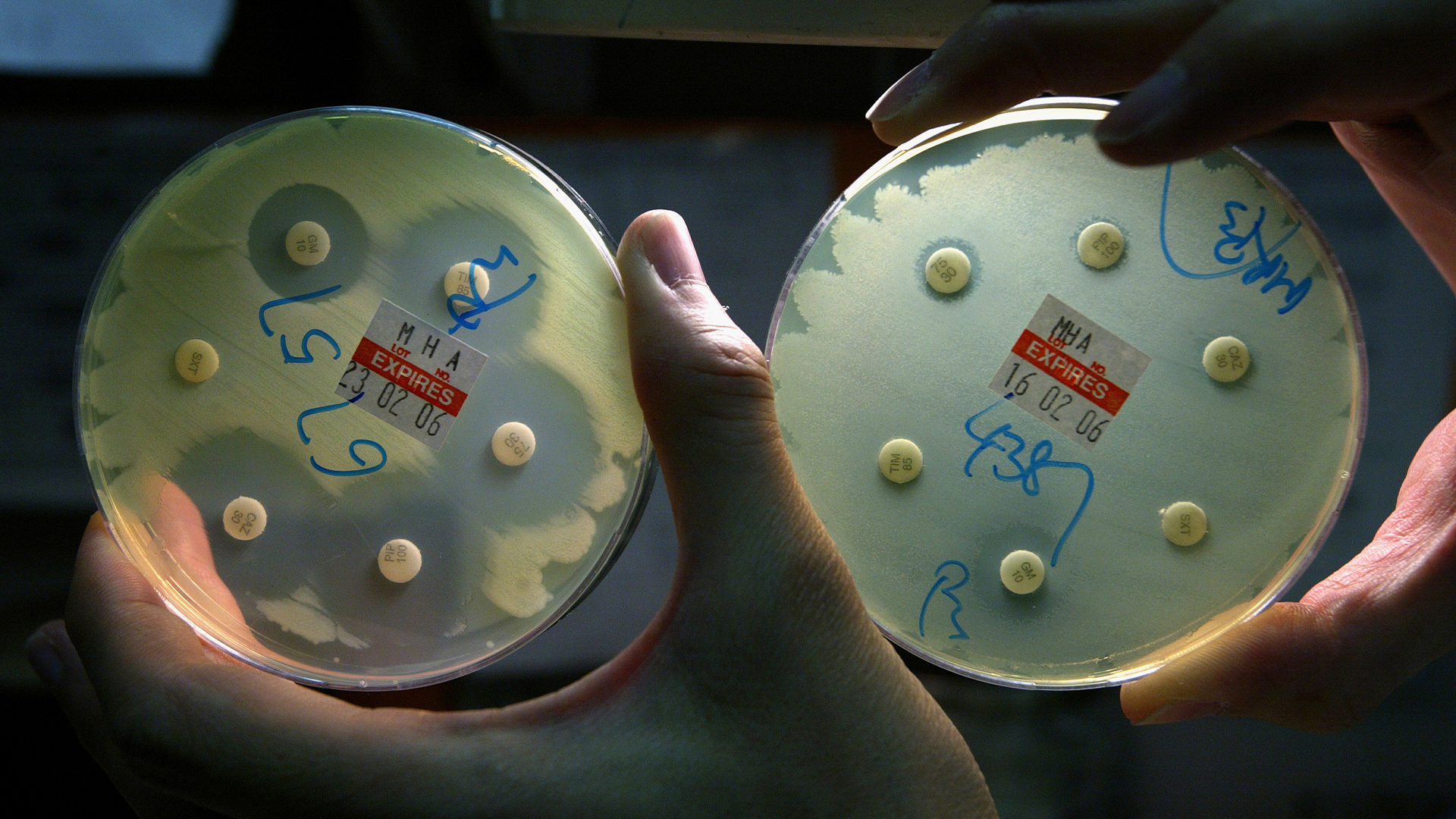
Antibiotics treat infections caused by bacteria — but bacteria can evolve to resist these essential drugs. Over time, the misuse of antibiotics has rapidly accelerated the spread of such resistant germs, and widely used antibiotics are becoming less effective.
So until alternative drugs to antibiotics are developed, how can we slow the rise of bacterial "superbugs"? The answer: Antibiotic stewardship.
Antibiotic stewardship aims to curb the misuse of antibiotics that drives bacteria to develop resistance in the first place. It involves setting clear principles for how doctors prescribe antibiotics and how patients use them in different settings, such as hospitals and nursing homes, and closely tracking whether those principles are followed, according to the Centers for Disease Control and Prevention (CDC).
The goal is to deter doctors from prescribing antibiotics for viral infections, which the drugs can't treat, or giving a patient a "broad-spectrum" antibiotic capable of killing many bacteria when a "narrow-spectrum" drug would work, for example. Broad-spectrum antibiotics pose a particular problem because they push a wide range of bacteria in the body to evolve resistance. And once they pick up new tools of resistance, the drug-resistant bugs can easily share them with a slew of additional bacteria.
Related: Dangerous 'superbugs' are a growing threat, and antibiotics can't stop their rise. What can?
"When we give lots of antibiotics, or we give more broad antibiotics than are necessary, then you will breed more antibiotic resistance in a patient and in our populations," Dr. Shruti Gohil, a lead investigator of four INSPIRE-ASP Trials — federally funded research aimed at curbing the overuse of antibiotics in hospitals — told Live Science.
The hope is that, by reigning in the misuse of antibiotics, we can reduce the rate that people get infected with multidrug-resistant organisms (MDROs), while also reducing the opportunities for new MDROs to emerge and spread between people.
Get the world’s most fascinating discoveries delivered straight to your inbox.
"That would be the metric we would all want to see happening, is a reversal of the prevalence of MDROs," said Gohil, who is also the associate medical director of the Epidemiology and Infection Prevention program at the University of California, Irvine School of Medicine.
How do we do that? There are a wide range of strategies.
One core strategy, of course, is to educate clinicians about how to use antibiotics appropriately, Gohil said. And to double-check physicians' work, hospitals can use "deescalation," which involves checking a patient's initial prescriptions to see whether a narrower, shorter or lower-dose antibiotics course would be more appropriate. Adjustments are made quickly if a different antibiotic is deemed to be a better choice than the one a doctor first picked. "That is currently the mainstay of antibiotic stewardship in the hospital," she said.
Another common strategy in U.S. hospitals is to provide guidance on the best antibiotics to use for different infections within the electronic systems that doctors use to order drugs. Hospitals may also restrict access to super-broad-spectrum drugs, requiring doctors to seek additional approval to use them, she added.
Speeding up diagnosis is another way to help doctors pick the right antibiotic, or skip one altogether for viral infections. When faced with a sick patient with a yet-undetermined diagnosis, clinicians often "err on the side of caution" by prescribing antibiotics that may not be needed, Gohil said. "That's one of the things that hobbles us when we're trying to make decisions, is that uncertainty."
Related: Could an antibiotic taken after unprotected sex prevent STIs? What to know about doxy-PEP.
The challenge is that even the very fastest diagnostic tests for bacterial infections take hours to deliver a result, she noted. And except in the case of bloodstream infections, those initial results can only say if a particular microbe is present in the body, not if it's driving the patient's illness. To reach a definitive diagnosis, doctors must combine these test results with what they can glean from the patient's symptoms and additional tests, such as chest X-rays.
In the future, faster diagnostics that deliver results at the bedside could ease this process, but they're far off. "I would not say it's impossible — I don't think it's imminent," Gohil said.
Beyond hospitals, clinicians at nursing homes and outpatient clinics, such as primary care practices and dentist offices, need similar education about and regulatory oversight over their use of antibiotics. And state and local health departments play a key role in coordinating and regulating those efforts.
Individual patients have a role to play as well — for example, by taking antibiotics as prescribed, not skipping doses and not taking antibiotics originally prescribed to other people, the CDC advises.
"Everybody who has bacterial resistance, for example, can shed and spread their bacteria. So by reducing individual risk, you anticipate that you will drop the overall population level risk," Gohil said of antibiotic stewardship programs. "By reducing antibiotic pressure on a given human, you reduce the likelihood that … that resistance will emerge."
And beyond the doctor's office, antibiotic stewardship is also critical in animal agriculture, because MDROs that arise in food-producing animals can make their way to humans.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus










