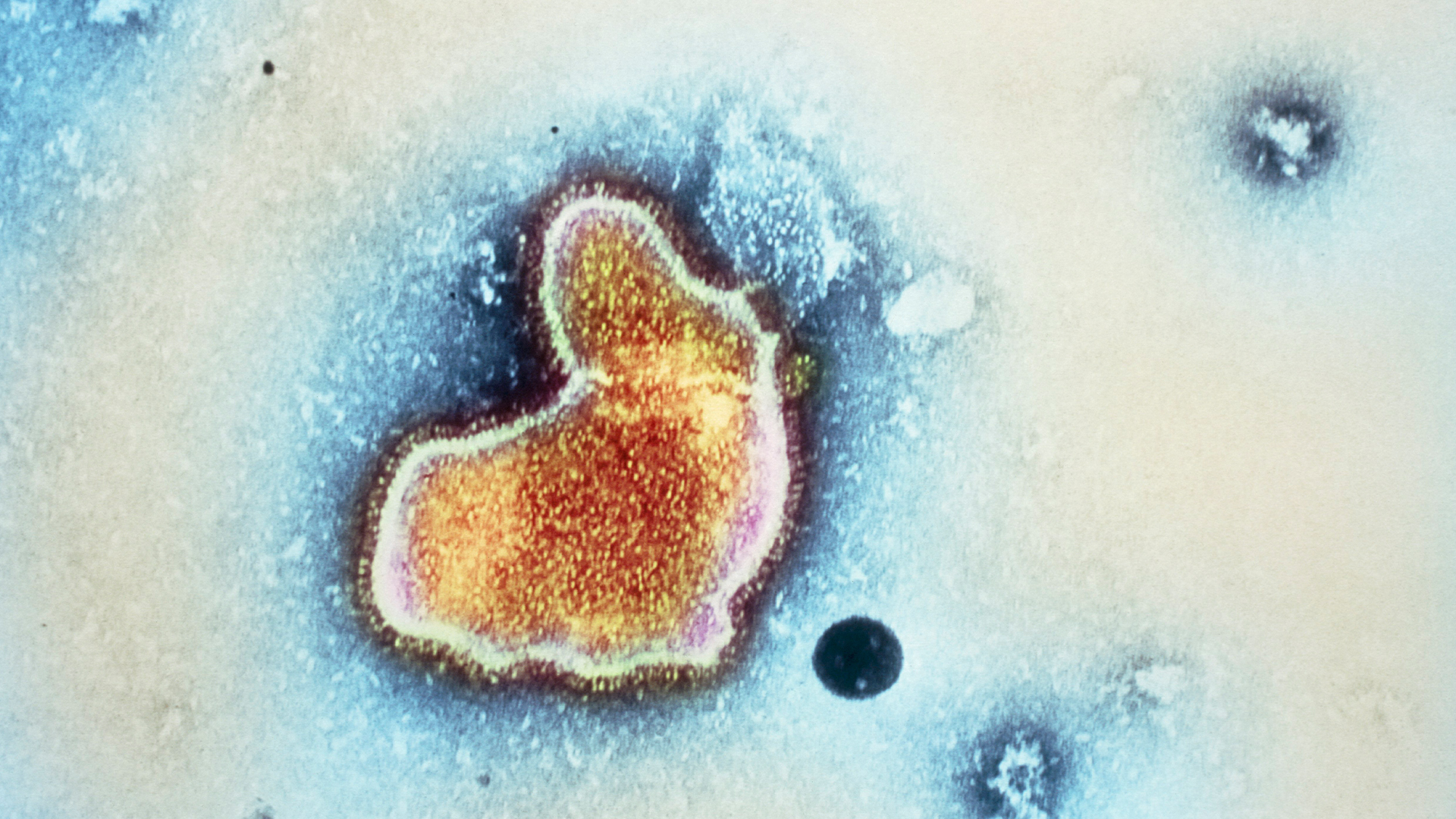
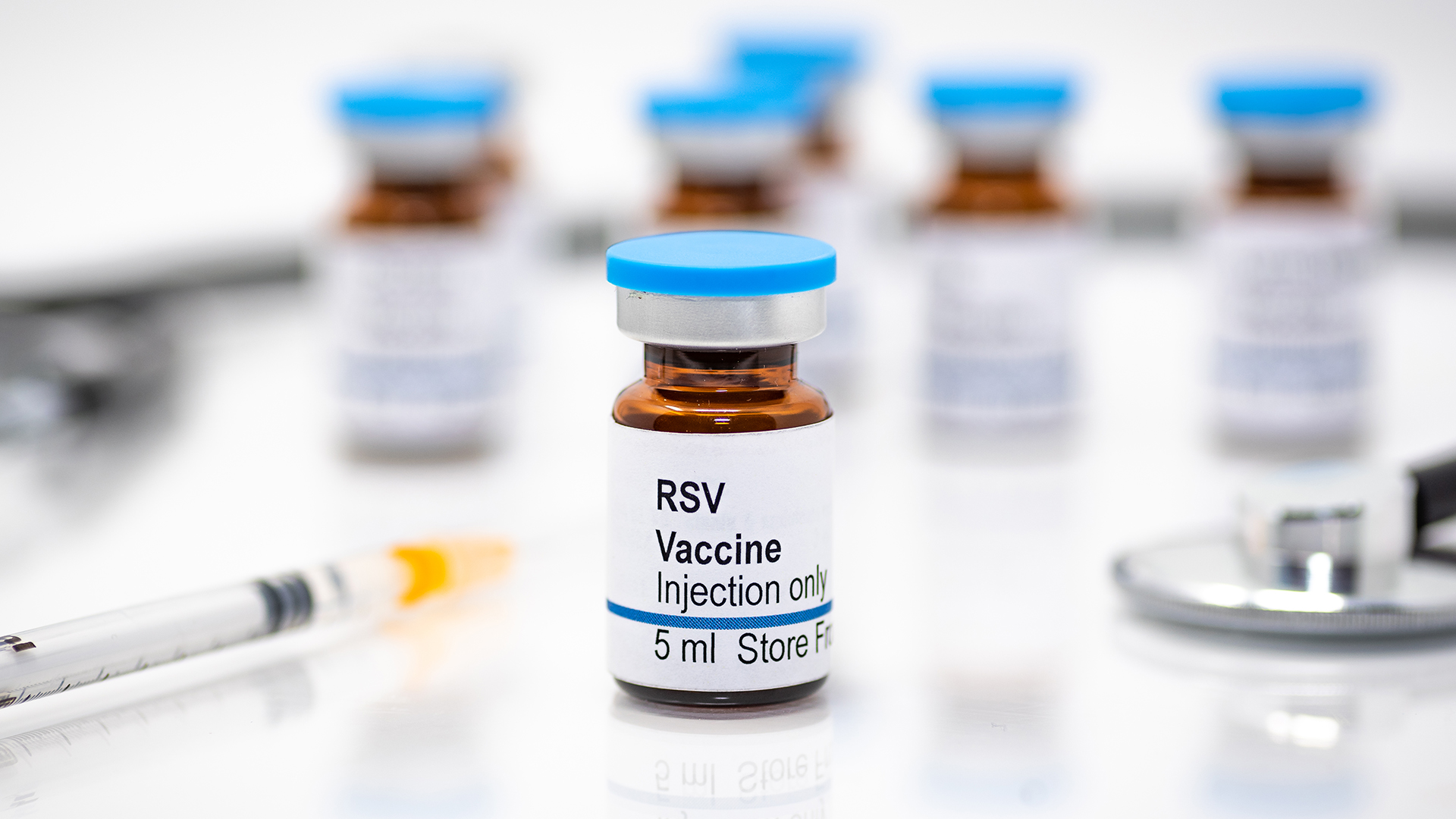
Who should get the new RSV vaccines? Here's everything you need to know
There are now RSV vaccines approved for older adults and for pregnant people, and antibody shots (not vaccines) available for babies. What's the difference?
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Respiratory syncytial virus, or RSV, is a common respiratory virus that spreads seasonally. A new drug and several vaccines that prevent the infection have recently come to market.
Here's what you need to know about the three vaccines and one injectable drug that were recently approved by the Food and Drug Administration (FDA) to fight RSV.
Related: Who should get the new COVID vaccines? What to know about the 2023-2024 shots
How serious is RSV?
Most people who contract RSV develop only cold-like symptoms, like a runny nose, decreased appetite, coughing and sneezing. Mild infections usually resolve without medical treatment. However, RSV can be severe and potentially fatal in infants, young children and older adults.
RSV season typically runs from fall to spring, although its timing has been disrupted in recent years due to the COVID-19 pandemic. Every year in the U.S., the virus leads to approximately 2.1 million doctor's visits among children younger than 5 and about 58,000 to 80,000 hospitalizations in the same age group, the CDC estimates. It's the most common cause of hospitalization in infants, and annually, 100 to 300 children under 5 die from the infection in the U.S.
The children at highest risk include premature babies, infants, children under age 2 who were born with heart disease or have chronic lung disease, and children with weakened immune systems or neuromuscular disorders.
Among adults ages 65 and older, RSV leads to 60,000 to 160,000 hospitalizations each year and about 6,000 to 10,000 deaths in the U.S. Older adults at the highest risk include those with chronic heart or lung disease, those with weakened immune systems, and those living in nursing homes or long-term care facilities. RSV infections in this age group can also worsen existing conditions, like chronic obstructive pulmonary disease (COPD) and congestive heart failure.
Is there a vaccine for RSV?
Yes, there are three FDA-approved vaccines for RSV. All three are approved for use in older adults, while one — called Abrysvo — is cleared for both older adults and pregnant people.
The FDA approved the first-ever RSV vaccine in May 2023. The shot, called Arexvy and made by the pharmaceutical company GSK, is approved for adults ages 60 and older.
The vaccine contains an adjuvant, a substance that revs up the immune system, and a lab-made version of a protein found on the surface of the virus. The virus uses this fusion, or "F" protein, to break into cells. The shot trains the immune system to recognize what the F protein looks like in its "prefusion" form, the shape it's in before it infects cells.
The second RSV vaccine approved by the FDA is called Abrysvo. Made by Pfizer, the shot was approved for older adults in May 2023 and for pregnant people in August 2023. Like Arexvy, Abrysvo contains lab-made prefusion F proteins. Neither vaccine contains preservatives, according to their drug labels.
The third vaccine is called mResvia and made by the company Moderna. Approved in May 2024, the shot is approved for use in adults ages 60 and older. Rather than carrying proteins, the vaccine contains a genetic molecule called mRNA that encodes instructions for human cells to build the prefusion F protein themselves.
How effective are the RSV vaccines?
In older adults, GSK's Arexvy and Pfizer's Abrysvo offer similar protection against RSV-associated "lower respiratory tract disease" (LRTD), meaning infections that affect the lungs.
In the first RSV season after vaccination, GSK's Arexvy lowers the chance of RSV-related LRTD by 82.6% and the risk of having to see a doctor for LRTD by 87.5%. A year after receiving one dose of vaccine, participants entered their second RSV season post-vaccination; data collected at that time showed that the shot was still 56.1% effective at preventing LRTD.
By comparison, in the first RSV season, Pfizer's Abrysvo is 88.9% protective against LRTD and 84.6% effective at preventing doctor's visits for LRTD. It's 78.6% protective against LRTD in the second season, based on data from part of a subsequent season, according to a Morbidity and Mortality Weekly Report (MMWR) from the CDC.
In February 2024, Pfizer released additional evidence that one dose of its vaccine is highly protective for two consecutive seasons in older adults.
When given in pregnancy, Abrysvo protects newborns by providing them with anti-RSV antibodies that cross the placenta and likely also crop up in breast milk. Abrysvo is specifically approved for use between week 32 and 36 of pregnancy; in clinical trials, this timing resulted in the most protection for newborns. The babies' risk of severe LRTD was slashed by 91.1% within 90 days of birth and by 76.5% within 180 days. The risk of LRTD of any severity was cut by 34.7% in the first 90 days and 57.3% in the first 180 days.
In trials with older adults, Moderna’s mResvia vaccine was nearly 79% protective against LRTD with two or more symptoms and about 81% protective against LRTD with three or more symptoms. This effectiveness waned over the course of months to 47% and 48%, respectively.
Who should get an RSV vaccine?
In summer 2024, the CDC updated its guidelines for which older adults should get an RSV vaccine.
It now says that all adults ages 75 and older should get one dose of any of the available vaccines. Younger adults ages 60 to 74 can also consider getting a shot if they have a high risk of contracting severe RSV infections. That would apply to people with chronic heart disease or weakened immune systems, as well as those who live in long-term care facilities such as nursing homes, for example.
At the time of this update, the CDC noted that the benefit of the vaccines may not outweigh the potential risks in people ages 60 to 74 with no major risk factors. That's because the Pfizer and GSK shots come with a slightly increased risk of the neurological syndrome called Guillain-Barré. This side effect is rare but still slightly more common among recipients of the vaccines than those in the same age range who haven't gotten them.
(As of August 2024, there aren't signals that the Moderna vaccine carries this same risk.)
Regarding using Abrysvo in pregnancy, the CDC has recommended that, during RSV season, pregnant people should get one dose of RSV vaccine between weeks 32 and 36 of gestation. The agency noted that most newborns likely need protection from the prenatal vaccine or from an antibody drug (as described later in the article), but not both.
"However, for example, if a baby is born less than two weeks after maternal immunization, then a doctor may recommend that the baby also receive the infant immunization," the CDC added.
Do you get vaccinated for RSV as a child?
No. There is no approved RSV vaccine for children.
However, newborns will get indirect protection in their early months of life if their parents receive an RSV vaccine during pregnancy. This protection comes from RSV-fighting antibodies that pass through the placenta. The shot doesn't result in a permanent "memory" of RSV in the child's immune system, like a vaccine given in childhood would.
Is there a drug to prevent RSV in children?
Yes, there are two antibody-based drugs available to protect infants from RSV.
Note that, if a pregnant person gets a prenatal RSV vaccine, their infant likely doesn't need an antibody drug on top of that, the CDC has said. But in select cases — for example, if a baby is born less than two weeks after maternal immunization — their doctor may also recommend an antibody drug.
In August 2023, the FDA approved a drug called Beyfortus (generic name nirsevimab-alip). The shot contains lab-made proteins that mimic antibodies made by the immune system. Called a monoclonal antibody, this type of drug doesn't train a child's immune system to make its own RSV-fighting antibodies, like a vaccine would. Instead it provides a ready-made supply. The one-time shot protects children for about five months, the length of an average RSV season, the CDC states.
The CDC temporarily tweaked some of its recommendations for the 2023-2024 RSV season, due to shortages of Beyfortus. But as of August 2024, the agency recommends one dose for all infants ages 8 months old and younger who are born during or entering their first RSV season.
It also recommends one dose for infants and children ages 8 to 19 months who are at increased risk for severe RSV disease and who are entering their second RSV season. This would include children with compromised immune systems, for instance.
Caregivers should consult a health care provider about which RSV antibody drug is available and right for their child.
In addition to Beyfortus, an older antibody shot against RSV, called Synagis (generic name palivizumab), is available. It was first approved in 1998. However, only children at the highest risk of severe disease, not healthy children, should be given this drug, the CDC recommends.
"Palivizumab is limited to children under 24 months of age with certain conditions that place them at high risk for severe RSV disease," the CDC states. "It must be given once a month during RSV season."
For example, Synagis is recommended for premature babies, especially those with chronic lung disease, and infants with certain heart diseases. This limited usage is partly due to the drug's high cost and the fact that it requires at least one and up to five shots a month during RSV season.
Editor's note: This article was last updated on Aug. 6, 2024, after the CDC updated its guidance for which older people should get an RSV vaccine. It was originally published on Aug. 24, 2023.
Get the world’s most fascinating discoveries delivered straight to your inbox.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others, or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus