FDA approves 1st RSV vaccine for use in pregnancy to stop infection in newborns
The FDA just approved an RSV vaccine that can be given in the third trimester of pregnancy.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
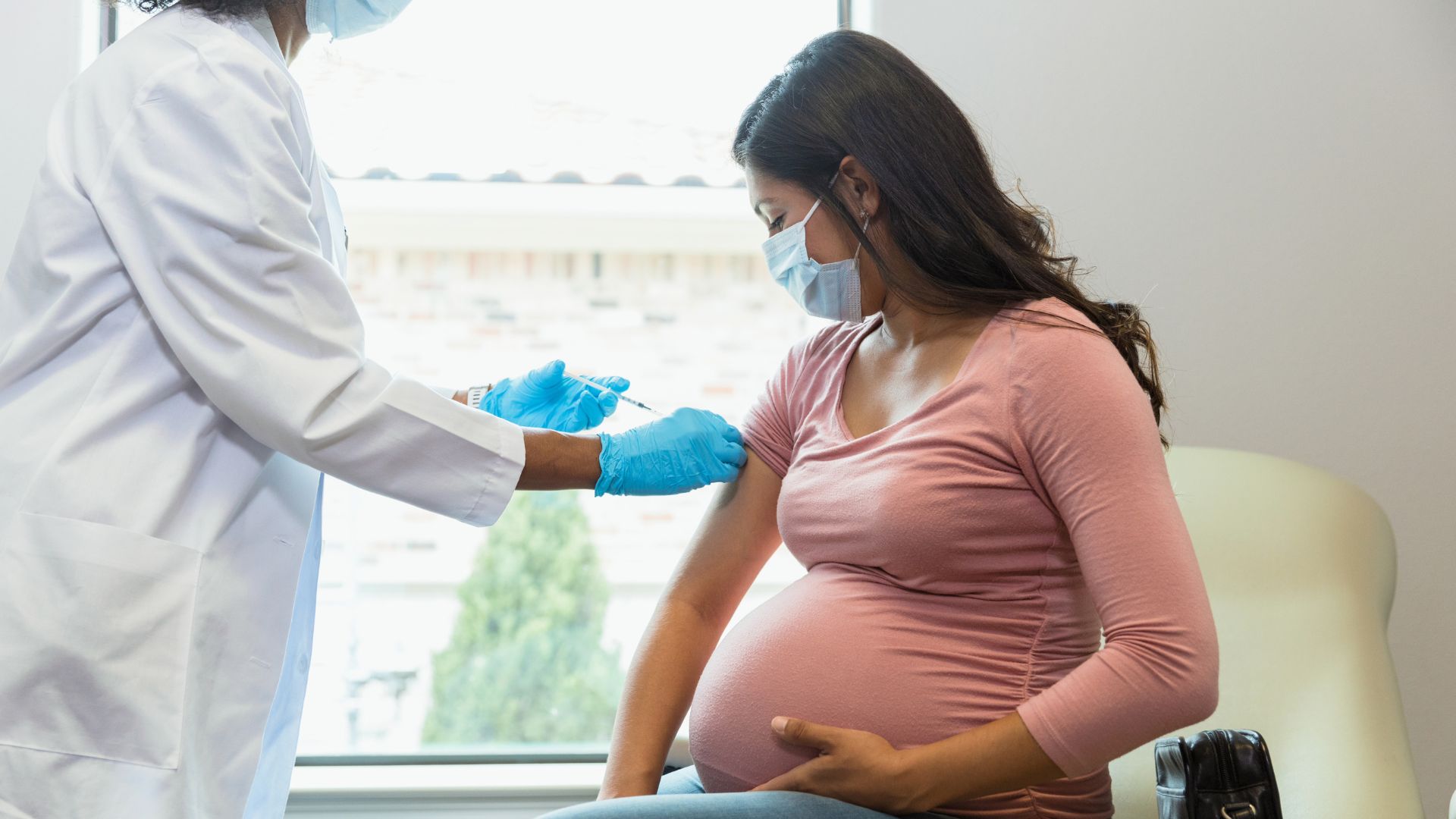
A respiratory syncytial virus (RSV) vaccine has just been approved for use in pregnant people that protects against dangerous infection in newborns.
On Monday (Aug. 21), the Food and Drug Administration (FDA) announced the approval of Abrysvo, a vaccine made by Pfizer, for use in pregnant individuals. The shot had already been cleared for use in people ages 60 and up, but now, it can also be given in the third trimester of pregnancy, specifically between weeks 32 and 36 of gestation.
The vaccine prompts the pregnant person's immune system to make protective antibodies that then pass to the fetus through the placenta. In addition, as with other vaccines given in pregnancy, the antibodies may also pass to newborns through normal breast milk and the nutrient-dense colostrum made just after birth. (Other vaccines recommended in pregnancy include the flu vaccine and Tdap vaccine for whooping cough, or pertussis.)
"RSV is a common cause of illness in children, and infants are among those at highest risk for severe disease, which can lead to hospitalization," Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, said in the announcement. "This approval provides an option for healthcare providers and pregnant individuals to protect infants from this potentially life-threatening disease."
Related: Having a baby: Stages of pregnancy by trimester
That said, now that the FDA has approved the shot, the public is still waiting on a Centers for Disease Control and Prevention (CDC) advisory committee to issue recommendations about who should get it. The CDC committee will likely meet in October, NBC reported.
In most people, RSV causes mild, cold-like illnesses that resolve without medical attention. But infants, young children and people older than 65 can develop severe disease and potentially die from the virus.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The FDA approved two RSV vaccines for older people earlier this year — Abrysvo and another vaccine called Arexvy — as well as an RSV-preventing antibody drug for infants up to 8 months old. Now, the new vaccine given in pregnancy provides an additional way to protect newborns and young infants from the infection.
In a clinical trial, 3,500 pregnant people received Abrysvo and another 3,500 received a placebo injection. In the 90 days after birth, the vaccinated group's babies had a 81.8% lower chance of severe lower respiratory tract disease — meaning severe lung infections — from RSV than the unvaccinated group's babies. And 180 days after birth, the vaccinated group's rate of severe infection was still 69.4% lower.
In a subgroup of 1,500 people who got vaccinated between weeks 32 and 36 of pregnancy, the shot worked even better. Their babies had a 91.1% lower rate of severe infection within 90 days and a 76.5% lower rate within 180, compared with a placebo group injected in the same trimester.
The most commonly reported side effects among the vaccinated people were pain at the injection site, headache, muscle pain and nausea.
Although not as commonly reported, the safety studies for the vaccines found that, compared with controls, vaccinated people had a slightly higher rate of the pregnancy-related high blood pressure disorder preeclampsia, the FDA noted. The vaccinated group also had a slightly higher risk of preterm birth.
With the data at hand, the researchers couldn't "establish or exclude a causal relationship between preterm birth and Abrysvo." For this reason, "the FDA is requiring the company to conduct postmarketing studies to assess the signal of serious risk of preterm birth and to assess hypertensive disorders of pregnancy, including pre-eclampsia."

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus





