Could vaccines prevent and treat Alzheimer's disease?
Scientists are testing vaccines as potential treatments for Alzheimer's, in an attempt to reign in harmful immune activity tied to the disease.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Despite decades of Alzheimer's research, scientists have not found a treatment that halts or dramatically slows the disease. Now, scientists are investigating if a completely new approach — so-called Alzheimer's vaccines — could alter the disease's course.
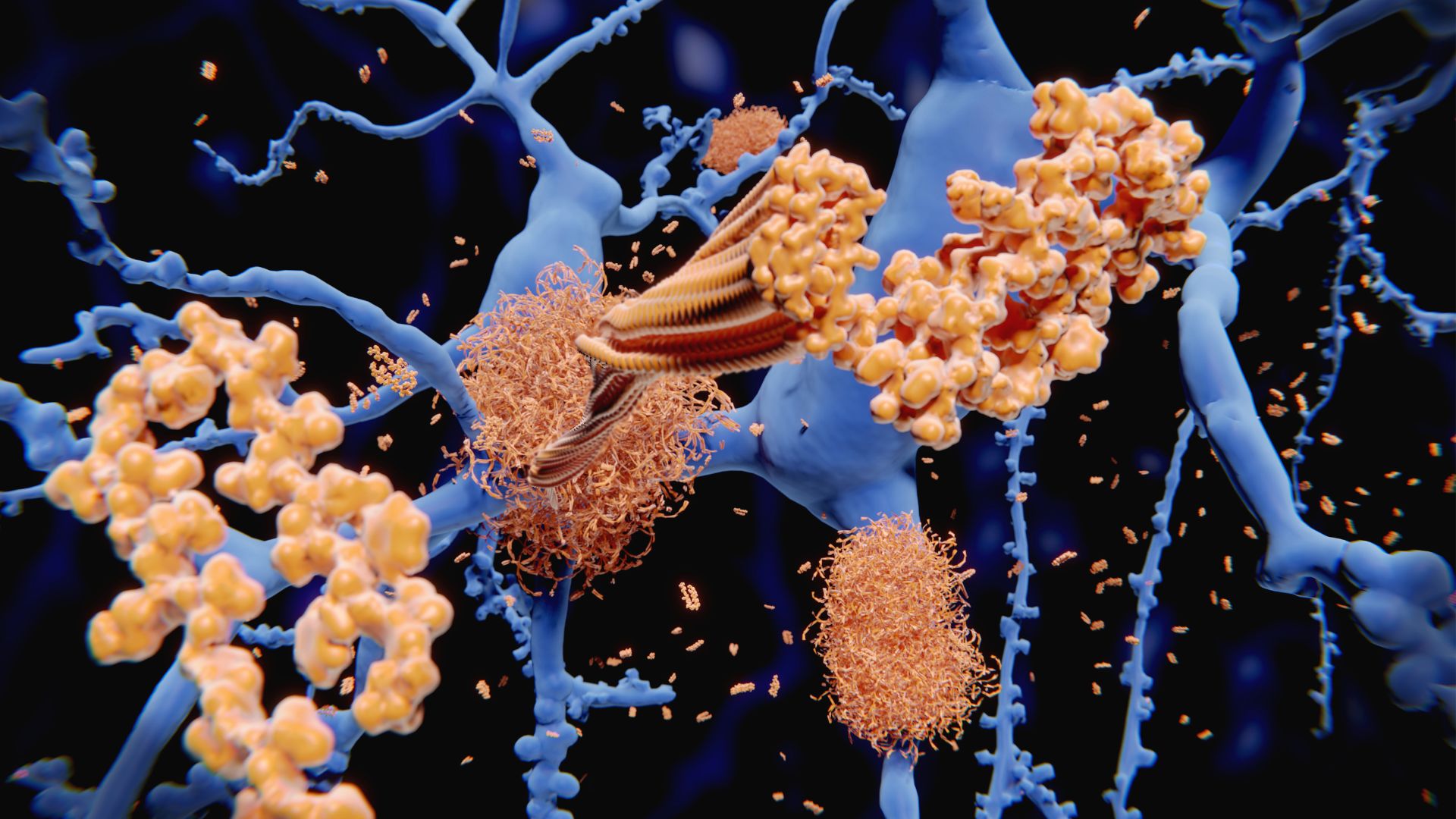
The logic behind this effort? Much of the previous research has placed beta-amyloid plaques, clumps of protein that accumulate in the brains of Alzheimer's patients, front-and-center. But drugs that remove beta-amyloid plaques alone don't seem to have much impact on the disease course. So some researchers have proposed that the plaques are a consequence, rather than a cause of Alzheimer's.
Instead, these researchers say, the disease may result from a badly tuned immune response to an initial bacterial or viral infection, in which the body overproduces inflammation and beta-amyloid while under-activating the cells that normally clear away those proteins away before they cause harm.
In this theory, inflammatory signals reach the brain, stimulating the production of beta-amyloid proteins, which may have antimicrobial properties intended to kill harmful invaders. However, when these proteins confuse healthy brain cells with microbes, they aggregate into plaques that damage the brain. Meanwhile, the cells that would normally mop up beta-amyloid — microglia — are underactive in Alzheimer's.
Related: Brain 'pacemaker' for Alzheimer's shows promise in slowing decline
This idea is bolstered by evidence that people infected with certain viruses, including the herpes virus and potentially SARS-CoV-2, face a heightened risk of later Alzheimer's disease.
Now, researchers are testing vaccines as potential Alzheimer's treatments, in an attempt to retrain the immune system to fight the disease. These vaccines would theoretically work by stimulating the cells that clear beta-amyloid while shutting down the immune cascade responsible for releasing the harmful protein. The shots could both be used to help prevent Alzheimer's and to treat the disease, once it's developed.
Get the world’s most fascinating discoveries delivered straight to your inbox.
One potential Alzheimer's vaccine has existed for a century.
The tuberculosis vaccine Bacille Calmette-Guérin (BCG) is routinely used in countries where tuberculosis remains a public health risk and is also administered after bladder cancer surgery to summon immune cells to attack lingering cancer cells. In two observational studies comprising thousands of patients, scientists noted that bladder cancer patients inoculated with the BCG vaccine have lower odds of developing Alzheimer's in the next several years. That raised the possibility that the vaccine helps prevent Alzheimer's, at least in the five years after inoculation.
One theory for why BCG might stave off Alzheimer's is that it retrains the immune system. The vaccine induces broad metabolic changes in different groups of immune cells. For example, immune cells called monocytes switched their energy source from glutamine to glucose, which is important for encoding immune memory. This makes immune responses faster while improving pathogen recognition.
The evidence that BCG is associated with a lower Alzheimer's risk and has long-term immune effects led Charles Greenblatt, a professor of microbiology and molecular genetics at the Hebrew University of Jerusalem and a leader of one of the observational studies, and his colleagues to test BCG as an Alzheimer's vaccine.
In a proof-of-concept study, the scientists administered BCG to 49 healthy participants around age 65. Nine months later, the researchers found that the vaccination affected the levels of different types of beta-amyloid in participants' blood plasma. The findings suggested that BCG recipients had lower levels of harmful amyloid clumps in their brains, suggestive of a protective effect.
Now, BCG is being tested in a small clinical trial of 15 adults with mild cognitive impairment and mild-to-moderate Alzheimer's, to see how it affects measurable signs of the conditions. But BCG isn't the only immune-modulating shot being tested in Alzheimer's.
Related: Shingles vaccine may protect against dementia
Dr. Tanuja Chitnis, a professor of neurology at Harvard Medical School and at Brigham and Women's Hospital, is leading the development of a nasal Alzheimer's vaccine. Called Protollin, the nasal spray was first developed in 2004 as a vaccine additive called an adjuvant, a substance that revs up the immune system.
Chitnis told Live Science that Protollin is made of bits of bacteria that are recognized by immune cells called macrophages. A 2008 mouse study suggests Protollin doesn't directly train the immune system to attack beta-amyloid but rather broadly activates immune cells so that they eat the proteins. In theory, Protollin could also make the immune cells more responsive to the bacteria and viruses thought to raise the risk of Alzheimer's in the first place.
Current Alzheimer's drugs, like aducanumab and lecanemab, deliver antibodies that specifically stick to and help clear amyloid plaques in the brain. However, the effectiveness of these drugs is highly debated, and they can come with side effects, including brain bleeds, swelling and atrophy. Protollin may avoid the side effects induced by a flood of antibodies, but that said, its safety has yet to be tested in large trials.
Meanwhile, several companies are developing Alzheimer's vaccines that directly target beta-amyloid. Vaxxinity’s UB-311 is the furthest along and its maker is preparing for late-stage clinical trials. Rather than jumpstarting a nonspecific immune response, like Protollin does, UB-311 works by training the body's immune cells to make the antibodies that recognize and clear beta-amyloid. Despite targeting beta-amyloid directly, the manufacturers report that there have been no cases of brain bleeds or swelling in the 43 trial participants — but again, larger trials will be needed to flag potential side effects.
One side effect to look out for is autoimmunity, James Nowick, a professor of chemistry at the University of California, Irvine who co-authored a review about the potential of Alzheimer's vaccines, told Live Science.
"Once the immune system is activated against an endogenous [body-made] peptide or protein, it is not simple to shut down the activation of the immune system," Nowick said. "A vaccine could thus produce a serious autoimmune response." It isn't as simple as turning inflammation on or off — in Alzheimer's, the microglia aren't active while other processes that release beta-amyloid might be hyperactive. An effective vaccine must work like an orchestra conductor, directing all the components of the brain's immune system in harmony.
In contrast to the approved antibodies, though, BCG and Protollin have a track-record of safety, as they're already approved for other conditions or as ingredients in other vaccines. But of course, beyond their safety, the effectiveness of these Alzheimer's vaccines also remains to be proven.
Even if vaccines work no better than existing monoclonal antibodies, though, they could make Alzheimer's treatment more accessible. Aducanumab and lecanemab require repeated infusions and cost patients $26,500 per year, while vaccines might cost only $1,000 per year.
"In early disease, a few doses may be sufficient to stop the process," Chitnis said. "In more established diseases, ongoing treatment may be required." And if the vaccines leave a lasting mark on immune cells, as seen in some studies of BCG, perhaps that could mean fewer doses or treatments will be required in the long-run.

Simon Spichak is a science journalist with an MSc in neuroscience, and he's a regular contributor to Being Patient, a news and community platform aimed at patients with Alzheimer's disease and dementia and their caregivers. In addition, his work has been featured in The Daily Beast, Health, Time, Futurism, TVO, and Fast Company, among other outlets. When he's not writing, he's drinking coffee, watching pigeons, and running a low-cost therapy clinic for students called Resolvve.
 Live Science Plus
Live Science Plus