RSV vaccine keeps older adults highly protected for at least 2 seasons
Pfizer's recently approved RSV vaccine maintains a high and fairly steady rate of effectiveness across consecutive RSV seasons.
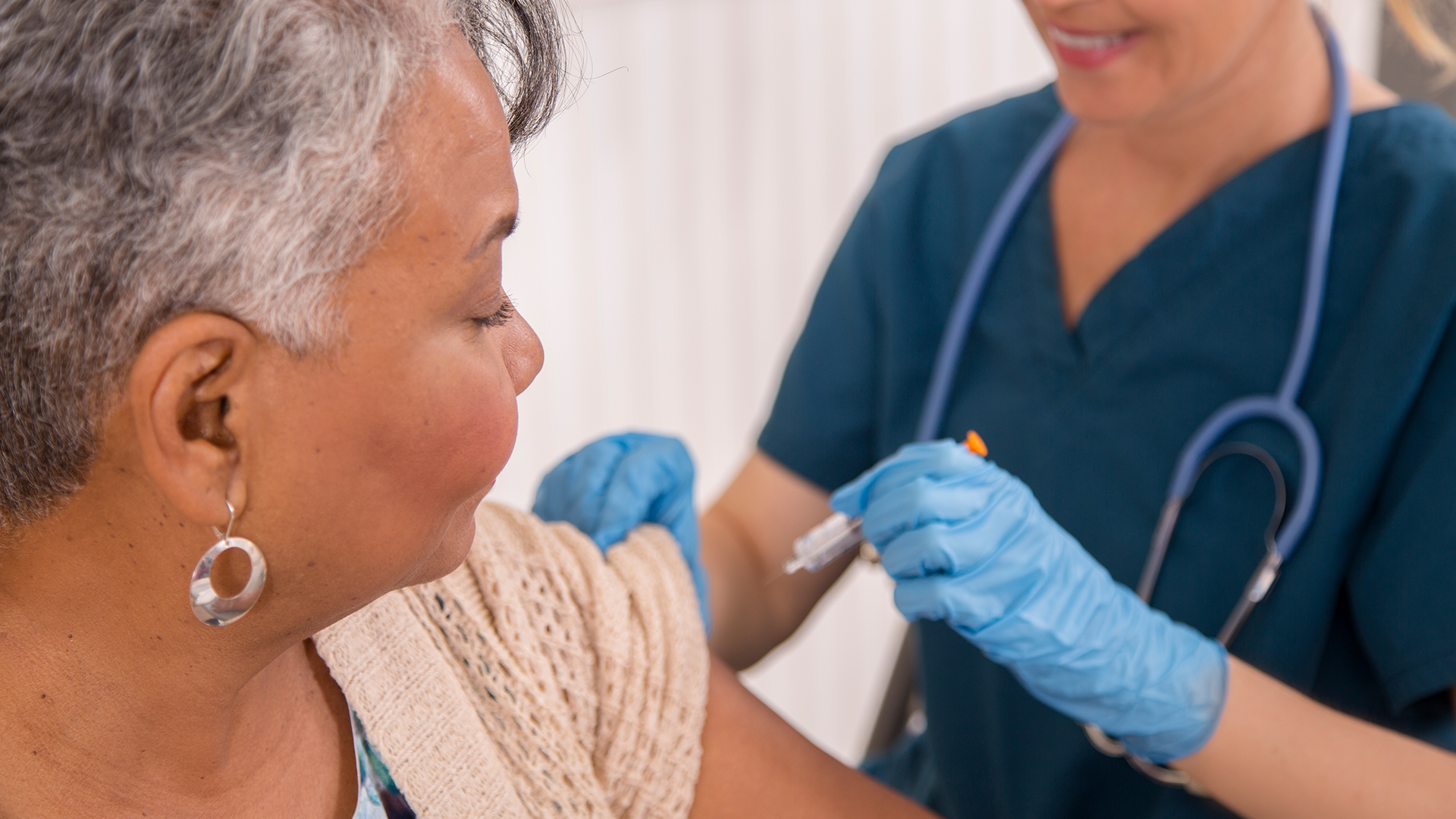
A Pfizer-made vaccine that protects older adults from respiratory syncytial virus (RSV) is highly effective for two consecutive seasons, new data show.
RSV is a common respiratory virus that typically spreads between fall and spring, and it hits adults over age 65 particularly hard. In this age group in the U.S., the virus causes 60,000 to 160,000 hospitalizations each year and about 6,000 to 10,000 deaths, according to the Centers for Disease Control and Prevention (CDC).
Last year, two RSV vaccines were approved for use in adults ages 60 and older: GSK's Arexvy and Pfizer's Abrysvo; the latter is also approved for pregnant people, whose newborns would be at risk of severe RSV without some form of protection. Now, new clinical-trial data released by Pfizer show that one dose of its vaccine "maintained consistently high protective efficacy" in older adults across two consecutive RSV seasons.
Related: Who should get the new RSV vaccines? Here's everything you need to know
The trial looked at how well the shot guards against "lower respiratory tract disease," or infections involving the lungs, Pfizer reported Thursday (Feb. 29). On that front, the vaccine was 88.9% effective in the first RSV season after administration and 77.8% effective in the second. The shot worked equally well against the two major subtypes of the virus: RSV A and RSV B.
The vaccine also showed decent protection against less-severe RSV infections, defined in the trial as triggering fewer symptoms than the more-severe cases, Pfizer reported. In this respect, the vaccine was 65.1% effective in the first season and 55.7% effective in the second.
These data will likely influence whether the CDC will recommend that older adults get RSV shots annually or every other year, CNBC reported.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The CDC hasn't recommended that all older adults get an RSV vaccine, whether Pfizer or GSK. Instead, the agency recommends that individuals discuss the risks and benefits of the shots with a doctor. That's partly because there were a handful of "inflammatory neurologic events" reported in trials of both vaccines. These events were rare among the thousands of people in the trials, but they included Guillain-Barré syndrome (GBS), a condition in which the immune system attacks the nerves in a way that can be life-threatening in severe cases.
New data presented Thursday by a CDC advisory committee show slightly higher rates of GBS with the Pfizer shot than with the GSK vaccine, STAT reported. However, cases of GBS were seen in recipients of both shots, and experts say it's too soon to know if the vaccines directly triggered those cases.
"Some of these data and findings are based on small numbers of cases and relatively small numbers of doses administered," Dr. Tom Shimabukuro, former director of the CDC's immunization safety office, told STAT. "And due to uncertainties and limitations, based on these early data we can't establish if there is an increased risk for GBS after RSV vaccination in these individuals 60 and older."
GBS can cause muscle weakness, paralysis and changes in sensation, and it most commonly affects adults over 50; severe cases that affect a person's ability to breathe can be life-threatening, but mild cases sometimes cause only brief weakness.
Most cases manifest in the wake of a bacterial or viral infection. And some vaccines, such as the flu shot, have been tied to a very slightly increased risk of GBS. (For the flu shot, there might be one or two extra cases of GBS out of a million vaccinated people, compared with expected background rates of the condition in the population, according to STAT.)
As of mid-February, CDC analysts have flagged 23 cases of GBS among 9.5 million people who have been vaccinated with either the Pfizer or GSK vaccine, with 15 cases tied to Pfizer. However, the data are somewhat muddy, because 14 of the 23 people had also received a second shot — such as a flu or shingles vaccine — at the same time as their RSV shot.
Overall, both shots are still considered very safe, as their most common side effects are mild. Currently, the CDC is mulling over the new safety and effectiveness data in the interest of updating the recommendations for both vaccines. The updated recommendations are expected in the summer, CNBC reported.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus





