8-year-old with rare, fatal disease shows dramatic improvement on experimental treatment
A child with a rare genetic disease that affects mitochondria is the first person to receive a new experimental treatment for the potentially life-threatening condition.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
The 8-year-old boy declined rapidly over only a few months. In August 2023, he was running and playing soccer, but by September, involuntary muscle contractions took hold of both his ankles. By October, he'd lost the ability to run and play sports, and by late November, he fell down frequently enough that physical therapists advised his family to get him a wheelchair.
Genetic testing confirmed the cause of the child's sudden deterioration: He carried two mutant copies of a gene called HPDL. The HPDL protein helps make an antioxidant, called coenzyme Q10, that is critical to the functioning of mitochondria, the powerhouses of cells.
HDPL deficiencies are rare and can vary in severity depending on which mutations a person carries. In the boy's case, the telltale symptoms of muscle spasticity and paralysis didn't emerge until after his eighth birthday — but two of his siblings had died in infancy of a more severe form of the condition, marked by brain damage and seizures.
The child's parents feared he might meet the same fate, given that there were no available treatments.
"They were so scared when we met," said Dr. Claire Miller, a pediatric neurologist who specializes in movement disorders at NYU Langone Health. At that time, in November 2023, the child couldn't cross the hospital lobby without falling, and his condition grew noticeably worse from one week to the next.
But with the help of Miller, other NYU specialists and special permission from the Food and Drug Administration, the child started an experimental treatment for his condition that had never been tested in humans before. The treatment includes a molecule that likely works by helping the body bypass the lack of HDPL in order to make CoQ10, the researchers think.
Related: Scientists just discovered a single molecule that may treat rare, devastating mitochondrial diseases
Get the world’s most fascinating discoveries delivered straight to your inbox.
Within a month, the boy could walk more than half a mile (1 kilometer) through Central Park with his family. Fast-forward to today, and he's joined them on a 4-mile (6 km) hike and also operates the pedals of a go-kart by himself. His strength is returning, along with his energy and stamina.
"Even small improvements — like walking more easily or having more energy — mean a lot," his family, who wish to remain anonymous, told Live Science in an email. "It's reassuring to know the treatment is making a difference."
For now, the plan is to keep the child taking the oral medication daily, and so far, the regimen has not had any concerning side effects. The child's medical team hopes to test the treatment in more patients with these HPDL deficiencies and then in a broader group of patients with related conditions that affect CoQ10.
"For us, trying an experimental treatment gave our child a chance at a better life," the family told Live Science. "And we're grateful we took that chance."
From mouse to human
This first-in-human clinical trial was enabled by promising experiments in lab mice, conducted in the lab of Dr. Michael Pacold, an assistant professor in the Department of Radiation Oncology at the NYU Grossman School of Medicine and the Perlmutter Cancer Center. Some of these mouse experiments were described in a new paper published Wednesday (July 9) in the journal Nature, along with details of the one-patient trial.
Prior to their latest work, the Pacold lab had published a study helping to unpack one of the roles of HPDL in mitochondria. They found that protein fulfills the first step in a chain reaction that ends with the creation of the antioxidant CoQ10. In summary, HPDL turns a compound called 4-HMA into another called 4-HB, which is then used to build the "head" of CoQ10.
It's thought that CoQ10 helps mitochondria diffuse reactive molecules that would otherwise damage them, and it may also be crucial to the processes by which mitochondria make fuel, Pacold told Live Science. It also performs other functions in the body, particularly in cell membranes, and when people don't make enough, that deficiency can cause widespread issues, especially in energy-hungry organs like the brain, kidneys and muscles.
HPDL deficiencies also fall under the broader umbrella of CoQ10 deficiencies, because the protein helps to make the head of this antioxidant.
CoQ10 is available as a supplement, but very little makes it into the brain when patients take it this way. The reason is not fully understood, but it's known that the antioxidant is poorly absorbed in the gut, so little makes it to the bloodstream, and even less makes it across the brain's protective barrier because the molecule is large, Pacold said. It's also hydrophobic, or greasy, which likely impedes its movement through the body, he added.
For these reasons, scientists have been searching for other ways to boost the antioxidant's levels in the central nervous system and thus treat these patients. The pathway defined by Pacold and colleagues offered a clue as to how to do that.
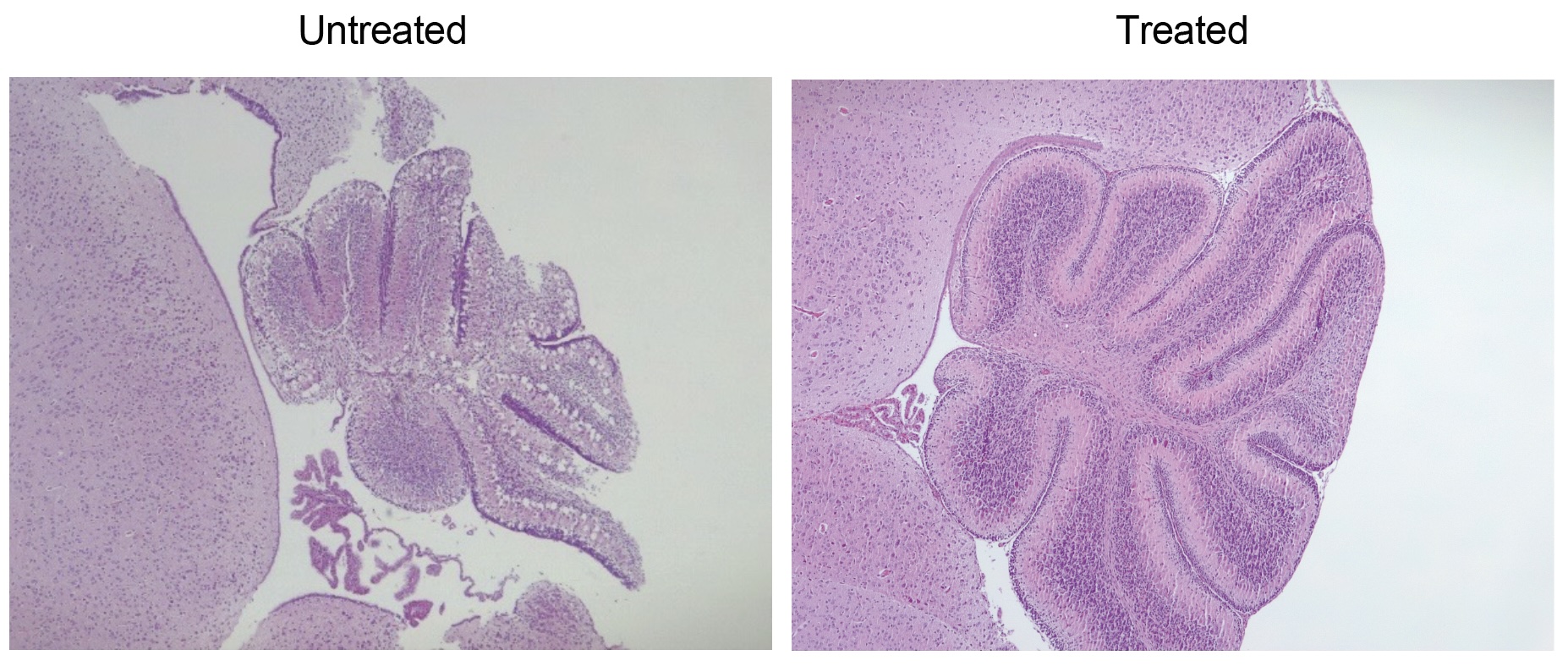
They tested their idea in genetically engineered lab mice that do not make HPDL. At baseline, these mice develop severe seizures and die within 15 days of birth. Treating the mice orally with CoQ10 does not save them.
So instead, the team tried giving the mice 4-HMA and 4-HB, so they essentially bypassed the missing domino in the chain. If treated early enough after birth, 90% of the mice given this treatment survived as long as normal lab mice — upward of 18 months — and they showed only limited neurological symptoms, such as paw weakness.
"His data was incredibly convincing to me," Miller said of Pacold's findings. Seeing the benefits of the treatment and the lack of side effects in mice, Miller agreed there was a chance that the same molecule could work in a person with HPDL deficiency — namely, the 8-year-old patient referred to their clinic.
"It seemed that the risk of trying this, although in a way unquantifiable, was also low," she said. "And the benefit was possibly huge, with the caveat that sometimes animal models don't necessarily translate well into humans."
More to learn
Like the lab mice, the 8-year-old child took a daily treatment orally in a solution dissolved in water. The patient was treated with only 4-HB, in part because the compound was already available in a "highly pure form," the researchers noted in their report.
The child says the medicine is sour and tastes better cold, Miller noted, but on the whole, it's fairly palatable. Having started the treatment in December 2023, now, "he practically is able to do all daily functional activities by himself except sports," his family told Live Science.
Taken together, the study and trial findings suggest that the treatment can restore function and boost survival in both mice and humans that lack HDPL, Siegfried Hekimi, a professor in the Department of Biology at McGill University who was not involved in the research, told Live Science in an email.
However, based on the available data, Hekimi questions whether the improvements seen in the lab mice and the 8-year-old boy are directly related to restored CoQ10 levels or to some other mechanism. It may be that the increases in 4-HMA and 4-HB have their own effects, independent of CoQ10, that are not yet fully understood and actually explain the medicine's therapeutic effect. At this point, it would be speculative to suggest that this same treatment might treat other types of CoQ10 deficiencies, Hekimi argued.
The study authors agreed that more research is needed to understand exactly how the experimental treatment works.
Pacold emphasized that this experimental treatment has so far been tried in only one patient. "The immediate next step is to try to get this into more patients with this disease … and establish whether or not the response that we saw in this child is typical," he said.
For now, though, Miller sees reason for hope.
"We all know that research does not always turn out as one hopes, and life does not turn out as one hopes," Miller said. But "this is a really happy story. This is a heartwarming story. This is a child who appeared to become very, very sick with symptoms in a very short amount of time, and he's doing so much better."
This article is for informational purposes only and is not meant to offer medical advice.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus










