States of matter: Definition and phases of change
The four fundamental states of matter are solid, liquid, gas and plasma, but there others, such as Bose-Einstein condensates and time crystals, that are man-made.

The phrase five states of matter is a term to describe everything that makes up the "stuff" in the universe — anything that takes up space and has mass is matter. But that phrase is actually outdated, as there are many more states of matter than that. Four of these occur naturally, while others are only made fleetingly in the lab, under extreme conditions.
All matter is made up of atoms, which are in turn made up of protons, neutrons and electrons.
Atoms come together to form molecules, which are the building blocks for all types of matter, according to Washington State University. Both atoms and molecules are held together by a form of potential energy called chemical energy, according to the U.S. Energy Information Administration.
Related: How many atoms are in the observable universe?
The four natural states of matter are: Solids, liquids, gases and plasma. Bose-Einstein condensates, however, are only made in the lab. Other exotic states of matter can also be manufactured under extreme conditions in a lab, such as fermionic condensates and time crystals. There's even a strange type of matter, known as a chain-melted state, that stably exists as both a solid and liquid at once.
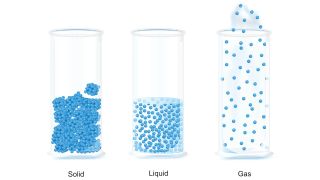
Solids, liquids and gas
In a solid, particles are packed tightly together so they don't move much. The electrons of each atom are constantly in motion, so the atoms have a small vibration, but they are fixed in their position. Because of this, particles in a solid have very low kinetic energy.
Solids have a definite shape, as well as mass and volume, and do not conform to the shape of the container in which they are placed. Solids also have a high density, meaning that the particles are tightly packed together.
In a liquid, the particles are more loosely packed than in a solid and are able to flow around each other, giving the liquid an indefinite shape. Therefore, the liquid will conform to the shape of its container.
Much like solids, liquids (most of which have a lower density than solids) are incredibly difficult to compress.
In a gas, the particles have a great deal of space between them and have high kinetic energy. A gas has no definite shape or volume. If unconfined, the particles of a gas will spread out indefinitely; if confined, the gas will expand to fill its container. When a gas is put under pressure by reducing the volume of the container, the space between particles is reduced and the gas is compressed, according to NASA's Glenn Research Center.
Plasma
Plasma is not a common state of matter here on Earth, but it may be the most common state of matter in the universe, according to the Jefferson Laboratory. Stars like the sun are essentially superheated balls of plasma.
Plasma consists of highly charged particles with extremely high kinetic energy. The noble gases (helium, neon, argon, krypton, xenon and radon) are often used to make glowing signs by using electricity to ionize them to the plasma state.
Bose-Einstein condensate
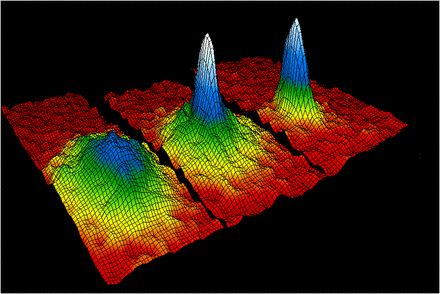
A BEC was first created by scientists in 1995. Using a combination of lasers and magnets, Eric Cornell and Carl Weiman, scientists at the Joint Institute for Lab Astrophysics (JILA) in Boulder, Colorado, cooled a sample of rubidium to within a few degrees of absolute zero. At this extremely low temperature, molecular motion comes very close to stopping. Since there is almost no kinetic energy being transferred from one atom to another, the atoms begin to clump together. There are no longer thousands of separate atoms, just one "super atom."
BECs are used to study quantum mechanics on a macroscopic level. Light appears to slow down as it passes through a BEC, allowing scientists to study the particle/wave paradox. A BEC also has many of the properties of a superfluid, or a fluid that flows without friction. BECs are also used to simulate conditions that might exist in black holes.
New states of matter
Many other states of matter have been created under extreme or exotic conditions. For instance, in May 2023, scientists created a "bosonic correlated insulator," or a symmetric crystalline state with a neutral charge. In 2021, scientists smooshed water to ultrahigh pressures and blasted it with lasers to create "superionic ice," or a strange new form of H20 similar to a solid oxygen lattice sitting in an ocean of floating hydrogen atoms. That same year, research published in the journal PNAS revealed that during the transformation between the state of liquid and solid, glass becomes a new state of matter referred to as liquid glass.
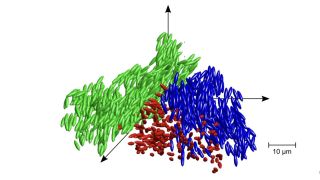
On a microscopic level, liquid glass is somewhere between a solid and a gel-like substance called a colloid — a mixture of particles that are larger than a single atom or molecule. When a substance transforms from a liquid to a solid, molecules are arranged in a crystalline structure — for glass, this doesn’t happen and particles are frozen in place before crystallisation occurs. The particles in liquid glass — however -are more flexible than solid glass, but can not rotate, according to the researchers.
"Our experiments provide the kind of evidence for the interplay between critical fluctuations and glassy arrest that the scientific community has been after for quite some time," senior author of the study and Professor of Soft Condensed Matter Theory at the University of Konstanz Matthias Fuchs, said in a statement.
Related: How do you weigh an atom?

Time crystals are a form of matter that were first proposed in 2012 by Nobel-prize winning physicist Frank Wilczek. Time crystals are made in the lab and have the ability to cycle between two states of energy without ever losing energy. Because they don't reach equilibrium or a steady state, they are able to dodge the second law of thermodynamics, which states that the disorder, or entropy, of a closed system, always increases.
Time crystals were created in a lab in 2017 and in 2021, Google announced that it had made a time crystal in a quantum computer, and that the crystal had lasted for 100 seconds before the ephemeral state disintegrated.
Fermionic condensates are another type of lab-made matter. A sister phase to the BEC, fermionic condensates were first created in 2004, according to NASA. Fermionic condensates are superfluids, meaning they can flow with no viscosity. Unlike BECs, they are made up of fermions, a type of matter that includes protons, neutrons and electrons with odd atomic numbers. Fermions normally like to be alone, but to create this matter phase, scientists have to coax them to pair up.
To do this, scientists make the matter very, very cold. In the first experiment to demonstrate this oddball phase, described in a 2003 study in the journal Physical Review Letters, scientists at JILA in Boulder, Colorado cooled a cloud of half a million potassium-40 atoms to less than a millionth of a degree above absolute zero, then applied a magnetic field to them. This forced the potassium atoms to pair up, creating a state akin to superconductivity that occurs in electron pairs.
How states of matter change
Adding or removing energy from matter causes a physical change as matter moves from one state to another. For example, adding thermal energy (heat) to liquid water causes it to become steam or vapor (a gas). And removing energy from liquid water causes it to become ice (a solid). Physical changes can also be caused by motion and pressure, according to the Abridged Science for High School Students by H.Messel.
Melting and freezing
When heat is applied to a solid, its particles begin to vibrate faster and move farther apart. When the substance reaches a certain combination of temperature and pressure, its melting point, the solid will begin to melt and turn into a liquid.

When two states of matter, such as solid and liquid, are at the equilibrium temperature and pressure, additional heat added into the system will not cause the overall temperature of the substance to increase until the entire sample reaches the same physical state, according to Encyclopaedia Britannica. For example, when you put ice into a glass of water and leave it out at room temperature, the ice and water will eventually come to the same temperature. As the ice melts from heat coming from the water, it will remain at 32 degrees Fahrenheit (0 degrees Celsius) until the entire ice cube melts before continuing to warm.
When heat is removed from a liquid, its particles slow down and begin to settle in one location within the substance. When the substance reaches a cool enough temperature at a certain pressure, the freezing point, the liquid becomes a solid.
Sublimation
When a solid is converted directly into a gas without going through a liquid phase, the process is known as sublimation. This may occur either when the temperature of the sample is rapidly increased beyond the boiling point (flash vaporization) or when a substance is "freeze-dried" by cooling it under vacuum conditions so that the water in the substance undergoes sublimation and is removed from the sample, according to the U.S. Geological Survey. A few volatile substances will undergo sublimation at room temperature and pressure, such as frozen carbon dioxide, or dry ice.

Vaporization
Vaporization is the conversion of a liquid to a gas and can occur through either evaporation or boiling, according to Encyclopaedia Britannica.
Because the particles of a liquid are in constant motion, they frequently collide with each other. Each collision also causes energy to be transferred, and when enough energy is transferred to particles near the surface they may be knocked completely away from the sample as free gas particles. Liquids cool as they evaporate because the energy transferred to surface molecules, which causes their escape, gets carried away with them.
Liquid boils when enough heat is added to a liquid to cause vapor bubbles to form below the surface. This boiling point is the temperature and pressure at which a liquid becomes a gas.
Condensation and deposition
Condensation occurs when a gas loses energy and comes together to form a liquid, according to the U.S. Geological Survey. For example, water vapor condenses into liquid water, known as its dew point.
Deposition occurs when a gas transforms directly into a solid, without going through the liquid phase. Water vapor becomes ice or frost when the air touching a solid, such as a blade of grass, is cooler than the rest of the air.
Additional resources
- Watch: Creation of a Bose-Einstein condensate, from the National Institute of Standards and Technology. Learn where the matter in the universe came from, from Cornell University's Ask an Astronomer.Read more about matter, elements and atoms, from Khan Academy.
This article was updated on Oct. 20, 2022 by Tia Ghose.
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
