Inside the 20-year quest to unravel the bizarre realm of 'quantum superchemistry'
More than two decades ago, scientists predicted that at ultra-low temperatures, many atoms could undergo 'quantum superchemistry' and chemically react as one. They've finally shown it's real.

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Chemistry depends on heat.
Atoms or molecules bounce around randomly, collide, and form other molecules. At higher temperatures, atoms collide more and the rate at which atoms become molecules increases. Below a certain temperature, the reaction won't happen at all.
But something very weird happens at the lowest temperatures. In this extreme cold, there is essentially no heat energy, yet chemical reactions happen faster than they do at high temperatures.
The phenomenon is called quantum superchemistry. And it was finally demonstrated last year, more than 20 years after physicists first proposed it.
In that experiment, University of Chicago physicist Cheng Chin and colleagues coaxed a group of cesium atoms at just a few nanokelvin into the same quantum state. Amazingly, each atom did not interact separately. Instead, 100,000 atoms reacted as one, almost instantaneously.
The first demonstration of this weird process has opened a window for scientists to better understand how chemical reactions operate in the strange realm of quantum mechanics, which governs the behavior of subatomic particles. It also may help to simulate quantum phenomena that classic computers struggle to model accurately, such as superconductivity.
But what happens after that, as with so many advances in research, is hard to predict. Chin, for one, has no plans to stop studying this strange form of chemistry.
Get the world’s most fascinating discoveries delivered straight to your inbox.
"No one knows how far we can go," Chin told Live Science. "It might take another 20 years. But nothing can stop us."
A new kind of chemistry
The term "superchemistry" was coined in 2000 to liken the phenomenon to other strange effects, like superconductivity and superfluidity, which emerge when large numbers of particles are in the same quantum state.
Unlike superconductivity or superfluidity, however, "'superchemistry' differs in that it is still barely realized, while these other phenomena have been extensively studied in experiments," Daniel Heinzen, lead author of the 2000 study and a physicist at the University of Texas at Austin, told Live Science in an email.
Heinzen and colleague Peter Drummond, who is now at the Swinburne University of Technology in Australia, were studying a special state of matter known as a Bose-Einstein condensate (BEC), in which atoms reach their lowest energy state and enter the same quantum state. In this regime, groups of atoms begin to act more like a single atom. At this small scale, particles can't be described as being in a given place or state. Rather, they have a probability of being in any given place or state, which is described by a mathematical equation known as the wave function.
In a BEC, just as Satyendra Nath Bose and Albert Einstein's work predicted, the individual wave functions of each atom become a single, collective wave function. Heinzen and Drummond realized that a group of particles with the same wave function is similar to a laser — a group of photons, or packets of light, that have the same wavelength. Unlike with other light sources, the peaks and troughs of a laser's wave are aligned. This allows its photons to stay focused in a tight beam over long distances, or to be broken up into bursts as short as millionths of a billionth of a second.
Related: How do lasers work?
Similarly, Heinzen, Drummond and their colleagues showed mathematically that the atoms in a BEC should behave in ways other groups of atoms don't. Near absolute zero, where there is almost no heat energy, quantum superchemistry means the atoms in a BEC could convert, quickly and all together, to molecules: Atoms A would bond in a flash to form molecules of A2, and so forth.
The process would resemble a phase transition, Chin says, such as when liquid water freezes to ice. And, thanks to the quantum weirdness of these systems, the more atoms condensed in the BEC, the faster the reaction happens, Heinzen and Drummond's calculations predicted.
The 20-year quest
Heinzen and his research group tried to demonstrate the phenomenon with experiments for several years. But they never found convincing evidence that the effect was happening. "And then we kind of dropped it," Heinzen said.
While Heinzen abandoned the quest to demonstrate quantum superchemistry, others were still hunting for ways to turn the wild theory into experimental reality. One of them was Chin, who started working on quantum superchemistry almost immediately.
Chin was a doctoral student studying cesium atoms at cold temperatures when Heinzen and Drummond's superchemistry paper came out. "My research was totally derailed because of this new research," Chin told Live Science. He set out on what would become a 20-year quest to achieve quantum superchemistry in the lab.
It wasn't a straight path, and Chin sometimes took breaks from working toward quantum superchemistry. But he never abandoned his goal.
"Nobody knew if this was going to work out before it happened. But also nobody said it couldn't happen," he said.
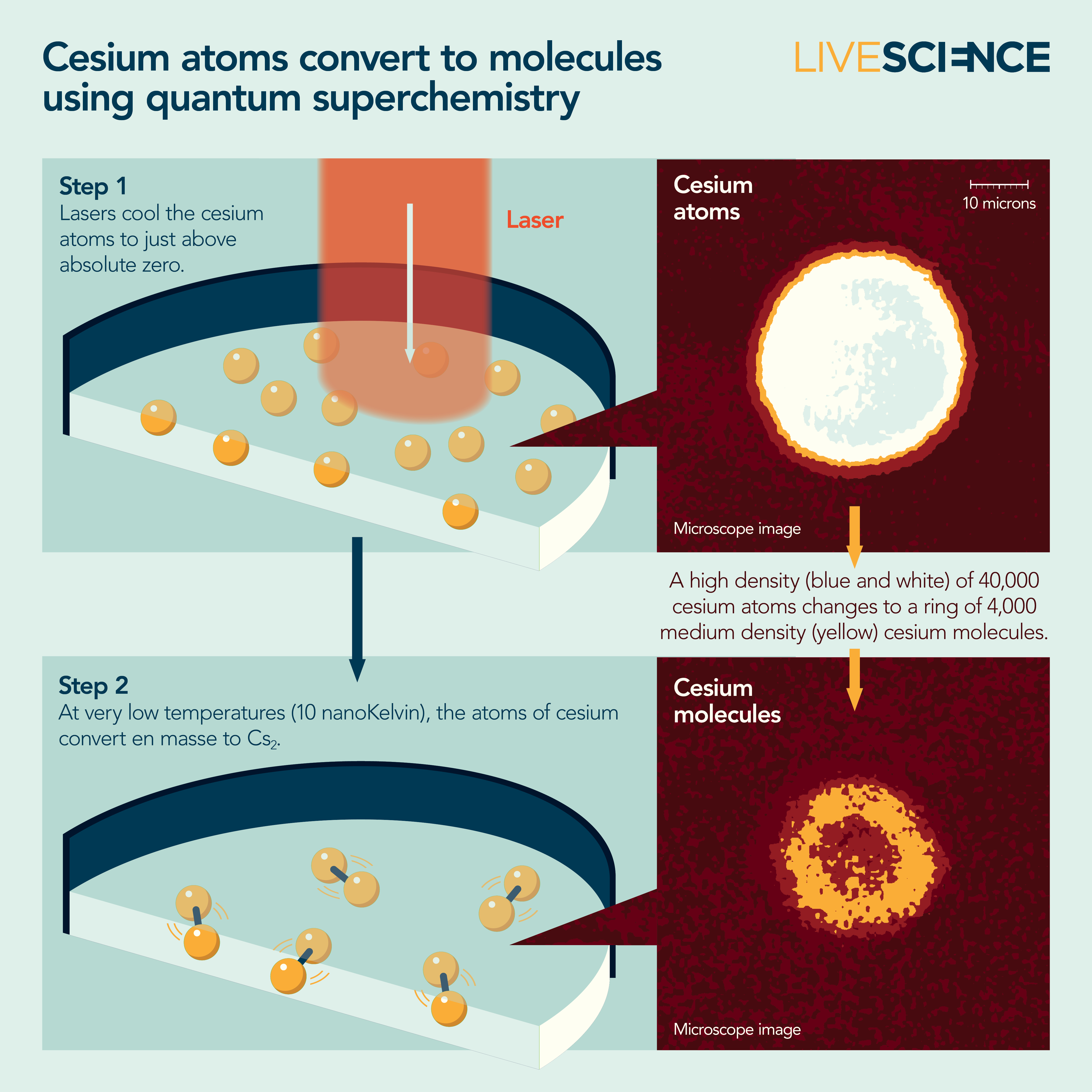
After a decade of slow progress, in 2010, Chin and his colleagues figured out how to precisely tune magnetic fields onto a BEC to coax cesium atoms together to make Cs2 molecules.
"That provided the evidence of how to move forward," Chin said.
But to show quantum superchemistry was occurring, his team still needed better ways to cool and control ultracold molecules.
Nobody knew if this was going to work out before it happened. But also nobody said it couldn't happen.
Cheng Chin, University of Chicago
Scientists typically use two techniques to push atoms and molecules to ultracold temperatures. First, lasers cool atoms to millionths of a kelvin above absolute zero. Atoms in the sample absorb photons from a laser tuned to very specific energy, thus reducing the atoms' momentum and the sample's temperature incrementally.
Next, they use evaporative cooling. The atoms in these experiments are trapped by laser light or magnetic fields. Scientists can adjust the traps to let the fastest — and, therefore, hottest — atoms escape. This process further cools the atoms to billionths of a kelvin, where quantum superchemistry is possible.
It was the second step that took Chin and his collaborators the longest to get right. For years, he had used bowl-shaped traps that pushed the atoms together in the middle, which raised the samples' temperature.
Six or seven years ago, his group began using a digital micromirror device to better control the shape of the trap. The result? Flat-bottomed traps, shaped something like petri dishes, where the atoms could spread out and stay ultracold.
Around 2020, Chin's group finally made a BEC of cesium molecules. They were some of the coldest molecules ever made, about ten-billionths of a degree above absolute zero. And while the team suspected quantum superchemistry had occurred, they didn't have proof.
That proof came three years later. By then, they had collected the evidence of two hallmarks of quantum superchemistry. First, the reaction was happening collectively, meaning many cesium atoms became cesium molecules at once. And second, it was reversible, meaning the atoms would become molecules, which would become atoms, and on and on.
For Chin, last year's experiments are just the beginning. They produced two-atom molecules using superchemistry. But Chin thinks three-atom molecules are within reach, and he's excited to see what else might be possible.
Where quantum superchemistry takes us
As is often the case in areas of fundamental research like this one, the experiments have raised new theoretical questions. For instance, in Heinzen and Drummond's theoretical quantum superchemistry system, more than half of all the atoms in a trap would convert into molecules and then go back again. But Chin's group observed that such a conversion happened only 20% of the time. “Much is still to be understood to gain higher efficiencies,” Chin said in an email.
Heinzen suspects collisions between molecules in the dense gas are to blame. Collisions could push molecules into different quantum states, knocking them out of the pool of condensed molecules. He and Drummond had not accounted for that possibility in their theory.
"It was obvious even from the beginning [that collisions were] going to be kind of a negative effect, but in 2000 we had no idea how big it would be," Heinzen said. "We just said, we're ignoring it because we don't know how big."
The experiments also revealed that three cesium atoms were frequently involved in forming a single Cs2 molecule (and leaving one Cs atom left over), which physicists call a three-body interaction. Previous predictions about quantum superchemistry did not include such interactions.
For Chin, that's a hint that he'll need to do some new experiments. If his group can design and perfect experiments to probe these many-body interactions, it could help elucidate the rules of quantum superchemistry.
Despite these open questions, many scientists view quantum superchemistry as a possible tool for better understanding chemical reactions in general. Atoms and molecules in a boiling beaker inhabit wide ranges of quantum states and interact in myriad ways that make them too complicated to study in fine detail experimentally. In contrast, atoms and very simple molecules in BECs are in precisely controlled, well-defined quantum states. So quantum superchemistry could be a way to study reactions in very fine detail.
"[It's] a very appealing regime in terms of advancing our fundamental understanding of chemistry," Waseem Bakr, a physicist at Princeton University who studies ultracold atoms and molecules, told Live Science.
Quantum superchemistry also has scientists excited because it provides precise control over molecular quantum states.
That could be useful for quantum simulation, a cousin of quantum computers. Typically, scientists simulate quantum systems on "classical" systems, such as conventional computers. But many processes, such as high-temperature superconduction, might be better modeled using quantum systems that are governed by the same quantum rules. Quantum superchemistry would give scientists a tool for producing molecules in specific quantum states that would enable those simulations, Bakr said.
Heinzen sees plenty of reasons for scientists to keep exploring the phenomenon he helped dream up more than 20 years ago. While the applications are little more than pipe dreams right now, history has shown that advances in fundamental science can sometimes lead to surprising applications down the road.
"It's not obvious right now," he said. "But it's still really worth doing."

Sam Lemonick is a freelance reporter and writer living in Maine. He studied chemistry and English literature at Carleton College in Minnesota. He focuses on chemistry, but has covered everything from machine learning to Mars, and mercury poisoning to Mason jars. He also reports for his local newspaper, the Harpswell Anchor.
 Live Science Plus
Live Science Plus