Quantum mechanics: Definitions, axioms, and key concepts of quantum physics
Quantum mechanics, or quantum physics, is the body of scientific laws that describe the wacky behavior of photons, electrons and the other subatomic particles that make up the universe.
At the smallest scales, the universe behaves very differently than the everyday world we observe around us. Quantum mechanics is the subfield of physics that describes this bizarre behavior of microscopic particles — atoms, electrons, photons and almost everything else in the molecular and submolecular realm.
Developed during the first half of the 20th century, the results of quantum mechanics are often extremely strange and counterintuitive. However, studying them has allowed physicists to reach a greater understanding about the nature of the universe, and could one day change the way we as humans process information.
How is quantum mechanics different from classical physics?
At the scale of atoms and electrons, many of the equations of classical mechanics, which describe the movement and interactions of things at everyday sizes and speeds, cease to be useful.
In classical mechanics, objects exist in a specific place at a specific time. In quantum mechanics, objects instead exist in a haze of probability; they have a certain chance of being at point A, another chance of being at point B and so on.
Who developed quantum mechanics?
Unlike Albert Einstein's famous theory of relativity, which was developed at roughly the same time, the origins of quantum mechanics cannot be attributed to a single scientist. Rather, multiple scientists contributed to a foundation that gradually gained acceptance and experimental verification between the late 1800s and 1930, according to the University of St. Andrews in Scotland.
In 1900, German physicist Max Planck was trying to explain why objects at specific temperatures, like the 1,470-degree-Fahrenheit (800 degrees Celsius) filament of a light bulb, glowed a specific color — in this case, red, according to the Perimeter Institute. Planck realized that equations used by physicist Ludwig Boltzmann to describe the behavior of gases could be translated into an explanation for this relationship between temperature and color. The problem was that Boltzmann's work relied on the fact that any given gas was made from tiny particles, meaning that light, too, was made from discrete bits.
This idea flew in the face of ideas about light at the time, when most physicists believed that light was a continuous wave and not a tiny packet. Planck himself didn't believe in either atoms or discrete bits of light, but his concept was given a boost in 1905, when Einstein published a paper, "Concerning an Heuristic Point of View Toward the Emission and Transformation of Light."
Get the world’s most fascinating discoveries delivered straight to your inbox.
Einstein envisioned light traveling not as a wave, but as some manner of "energy quanta." This packet of energy, Einstein suggested in his paper, could "be absorbed or generated only as a whole," specifically when an atom "jumps" between quantized vibration rates. This is where the "quantum" part of quantum mechanics comes from.
With this new way to conceive of light, Einstein offered insights into the behavior of nine phenomena in his paper, including the specific colors that Planck described being emitted from a light bulb filament. It also explained how certain colors of light could eject electrons off metal surfaces — a phenomenon known as the photoelectric effect.
What is wave-particle duality?
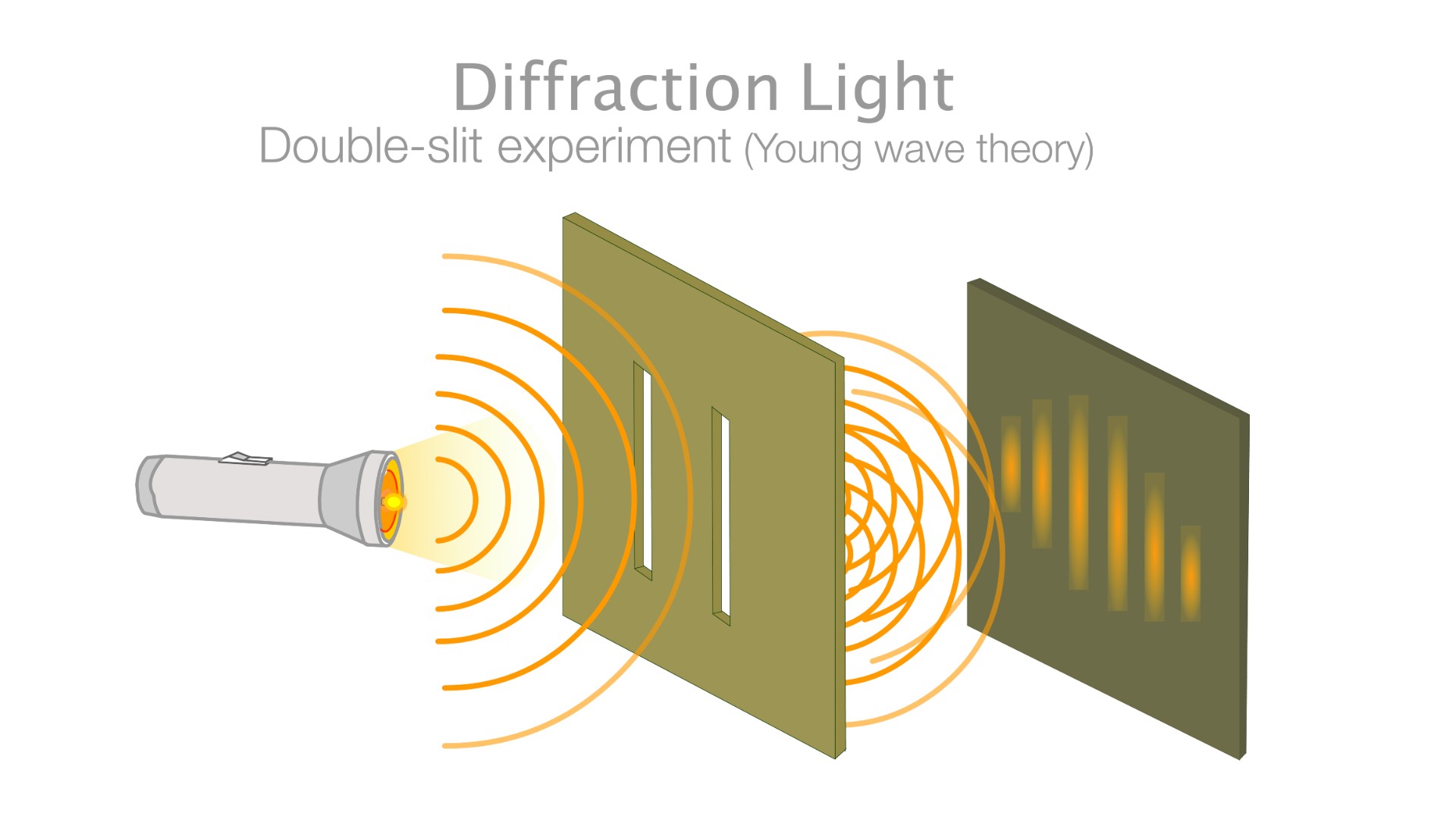
In quantum mechanics, particles can sometimes exist as waves and sometimes exist as particles. This can be most famously seen in the double-slit experiment, where particles such as electrons are shot at a board with two slits cut into it, behind which sits a screen that lights up when an electron hits it. If the electrons were particles, they would create two bright lines where they had impacted the screen after passing through one or the other of the slits, according to a popular article in Nature.
Instead, when the experiment is conducted, an interference pattern forms on the screen. This pattern of dark and bright bands makes sense only if the electrons are waves, with crests (high points) and troughs (low points), that can interfere with one another. Even when a single electron is shot through the slits at a time, the interference pattern shows up — an effect akin to a single electron interfering with itself.
In 1924, French physicist Louis de Broglie used the equations of Einstein's theory of special relativity to show that particles can exhibit wave-like characteristics and that waves can exhibit particle-like characteristics — a finding for which he won the Nobel Prize a few years later.
How does quantum mechanics describe atoms?
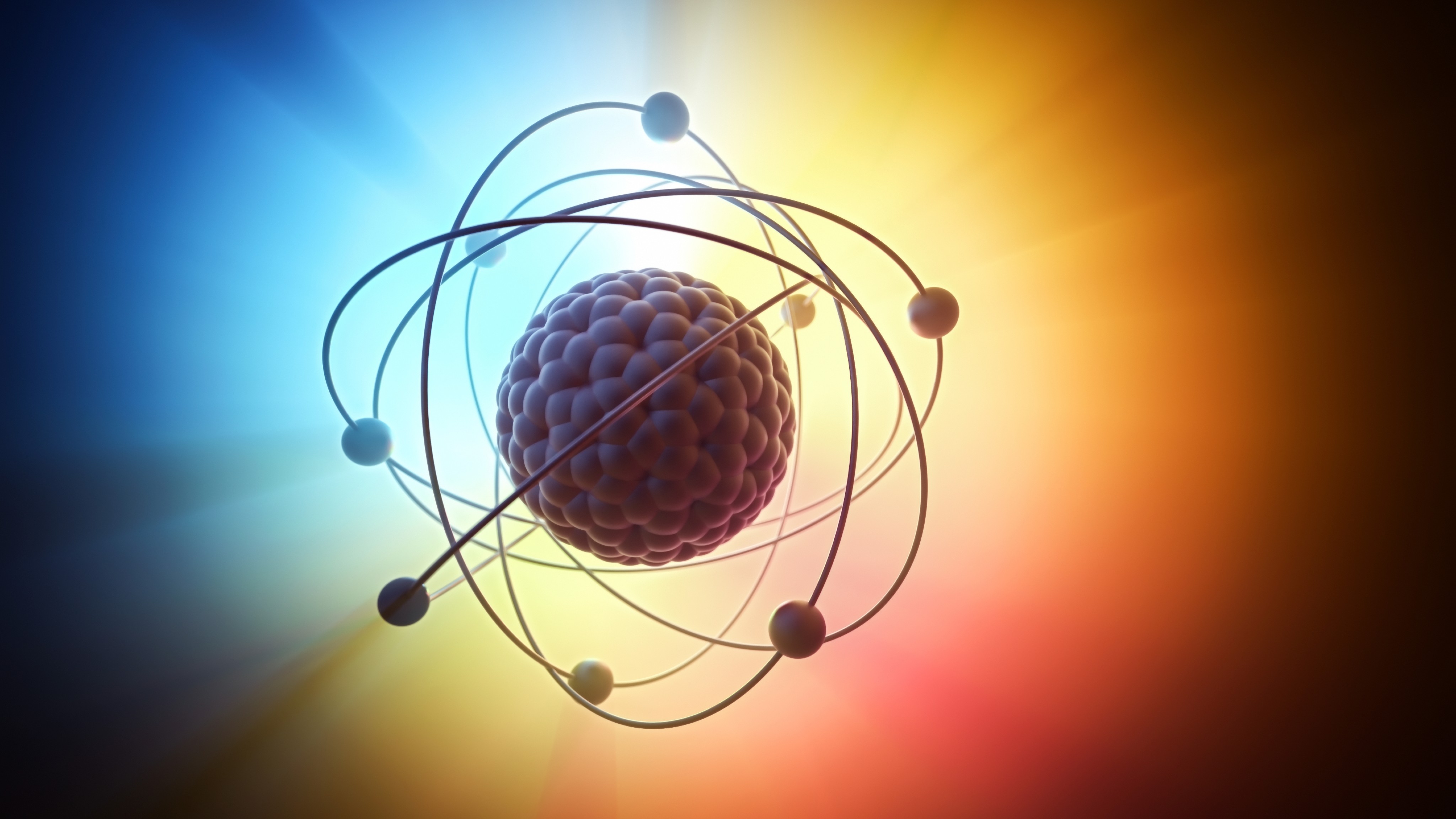
In the 1910s, Danish physicist Niels Bohr tried to describe the internal structure of atoms using quantum mechanics. By this point, it was known that an atom was made of a heavy, dense, positively charged nucleus surrounded by a swarm of tiny, light, negatively charged electrons. Bohr put the electrons into orbits around the nucleus, like planets in a subatomic solar system, except they could only have certain predefined orbital distances. By jumping from one orbit to another, the atom could receive or emit radiation at specific energies, reflecting their quantum nature.
Shortly afterward, two scientists, working independently and using separate lines of mathematical thinking, created a more complete quantum picture of the atom, according to the American Physical Society. In Germany, physicist Werner Heisenberg accomplished this by developing "matrix mechanics." Austrian-Irish physicist Erwin Schrödinger developed a similar theory called "wave mechanics." Schrödinger showed in 1926 that these two approaches were equivalent.
The Heisenberg-Schrödinger model of the atom, in which each electron acts as a wave around the nucleus of an atom, replaced the earlier Bohr model. In the Heisenberg-Schrödinger model of the atom, electrons obey a "wave function" and occupy "orbitals" rather than orbits. Unlike the circular orbits of the Bohr model, atomic orbitals have a variety of shapes, ranging from spheres to dumbbells to daisies, according to an explanatory website from chemist Jim Clark.
What is the Schrödinger's cat paradox?
Schrödinger's cat is an often-misunderstood thought experiment describing the qualms that some of the early developers of quantum mechanics had with its results. While Bohr and many of his students believed that quantum mechanics suggested that particles don't have well-defined properties until they are observed, Schrödinger and Einstein were unable to believe such a possibility because it would lead to ridiculous conclusions about the nature of reality.
In 1935, Schrödinger proposed an experiment in which the life or death of a cat would depend on the random flip of a quantum particle, whose state would remain unseen until a box was opened. Schrödinger hoped to show the absurdity of Bohr's ideas with a real-world example that depended on the probabilistic nature of a quantum particle but yielded a nonsensical result.
According to Bohr's interpretation of quantum mechanics, until the box was opened, the cat existed in the impossible dual position of being both alive and dead at the same time. (No actual cat has ever been subjected to this experiment.) Both Schrödinger and Einstein believed that this helped show that quantum mechanics was an incomplete theory and would eventually be superseded by one that accorded with ordinary experience.
Even today, physicists struggle to explain why subatomic particles can seemingly exist in a superposition of different states, but large structures — like the universe itself — seemingly do not. Proposed tweaks to Schrödinger's equations could help resolve this tension, but so far none have been widely accepted by the scientific community.
What is quantum entanglement?
Schrödinger and Einstein helped highlight another strange result of quantum mechanics that neither could fully fathom. In 1935, Einstein, along with physicists Boris Podolsky and Nathan Rosen, showed that two quantum particles can be set up so that their quantum states would always be correlated with one another, according to the Stanford Encyclopedia of Philosophy. The particles essentially always "knew" about each other's properties. That means that measuring the state of one particle would instantaneously tell you the state of its twin, no matter how far apart they were, a result that Einstein called "spooky action at a distance," but which Schrödinger soon dubbed "entanglement."
Entanglement has been shown to be one of the most essential aspects of quantum mechanics and occurs in the real world all the time. Researchers frequently conduct experiments using quantum entanglement and the phenomenon is part of the basis for the emerging field of quantum computing.
What is quantum computing?
Unlike classical computers that process data using binary bits, which can be in one of two states — 0 or 1 — quantum computers use particles such as electrons or photons. These quantum bits, or qubits, represent a superposition of both 0 and 1 — meaning they can exist in multiple states at once.
This superposition enables quantum computers to perform calculations in parallel by processing all states of a qubit at the same time. Furthermore, quantum entanglement allows multiple qubits to share information and interact simultaneously, regardless of the distance between particles.
While quantum superposition and entanglement make the processing potential of quantum computers much higher than classical computers, the field has a long way to go. Currently, quantum computers are too small, too difficult to maintain and too error-prone to compete with the best classical computers. However, many experts expect this will one day change as the field advances.
Are quantum mechanics and general relativity incompatible?
At the moment, physicists lack a full explanation for all observed particles and forces in the universe, which is often called a theory of everything. Einstein's relativity describes large and massive things, while quantum mechanics describes small and insubstantial things. The two theories are not exactly incompatible, but nobody knows how to make them fit together.
Many researchers have sought a theory of quantum gravity, which would introduce gravity into quantum mechanics and explain everything from the subatomic to the supergalactic realms. There are a great deal of proposals for how to do this, such as inventing a hypothetical quantum particle for gravity called the graviton, but so far, no single theory has been able to fit all observations of objects in our universe. Another popular proposal, string theory, which posits that the most fundamental entities are tiny strings vibrating in many dimensions, has started to become less widely accepted by physicists since little evidence in its favor has been discovered. Other researchers have also worked on theories involving loop quantum gravity, in which both time and space come in discrete, tiny chunks, but so far no one idea has managed to gain a major hold among the physics community.
This article was originally written by Live Science contributor Robert Coolman and was updated by Adam Mann on March 2, 2022. It was updated again by Brandon Specktor on April 29, 2024.
Bibliography
Bow, E. (2019, June 19). A quick quantum history of the light bulb. Inside the Perimeter https://insidetheperimeter.ca/quick-quantum-history-of-the-light-bulb/
Clark, J. (2021, May). Atomic orbitals. https://www.chemguide.co.uk/atoms/properties/atomorbs.html
Coolman, R. (2014, September 11). What is classical mechanics? Live Science. https://www.livescience.com/47814-classical-mechanics.html
O'Connor, J. J., & Robertson, E. F. (1996, May). A history of quantum mechanics. https://mathshistory.st-andrews.ac.uk/HistTopics/The_Quantum_age_begins/
Einstein, A. (1905). On a heuristic point of view concerning the production and transformation of light. Annals of Physics. https://einsteinpapers.press.princeton.edu/vol2-trans/100
Mann, A. (2020, February 28) Schrodinger’s cat: The favorite misunderstood pet of quantum mechanics. Live Science. https://www.livescience.com/schrodingers-cat.html
Mann, A. (2019, August 29) What is the theory of everything? Space.com. https://www.space.com/theory-of-everything-definition.html
Moskowitz, C. (2012, March 25). Largest molecules yet behave like waves in quantum double-slit experiment. Live Science. https://www.livescience.com/19268-quantum-double-slit-experiment-largest-molecules.html
Schirber, M. (2019, July 9). What is relativity? Live Science. https://www.livescience.com/32216-what-is-relativity.html
The Nobel Prize (n.d.). Louis de Broglie facts. https://www.nobelprize.org/prizes/physics/1929/broglie/facts/
Tretkoff, E. (2008, February). This month in physics history: February 1927 Heisenberg’s uncertainty principle. American Physical Society. https://www.aps.org/publications/apsnews/200802/physicshistory.cfm
Wood, C. (2019, August 27). What is quantum gravity? Space.com. https://www.space.com/quantum-gravity.html

Adam Mann is a freelance journalist with over a decade of experience, specializing in astronomy and physics stories. He has a bachelor's degree in astrophysics from UC Berkeley. His work has appeared in the New Yorker, New York Times, National Geographic, Wall Street Journal, Wired, Nature, Science, and many other places. He lives in Oakland, California, where he enjoys riding his bike.
- Robert CoolmanLive Science Contributor