
How a Cream for Genital Warts Might Also Help with Flu Pandemics
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Genital warts and the flu don't seem to have much in common, other than that they are both caused by viruses. But now, researchers are testing whether a cream that's commonly used to treat genital warts could also help boost the protection of flu vaccines in the event of a pandemic.
In a study that began earlier this summer, researchers at Baylor College of Medicine in Houston will look at whether the cream, called imiquimod, can improve people's immune responses to vaccines against H5N1, a potentially deadly strain of bird flu, according to the National Institutes of Health (NIH).
Currently, H5N1 is very rare in people and does not spread easily. But researchers are concerned that if the virus were to undergo certain genetic changes, it could spread more easily and cause a pandemic, the NIH said in a statement.
That's why scientists have already made a vaccine against H5N1, which is stored in the National Pre-Pandemic Influenza Vaccine Stockpile. But if there were a way to boost the vaccine's effectiveness, researchers could stretch the supply further and vaccinate more people in the event of a pandemic.
Enter imiquimod. The cream activates the part of the body's immune system that helps fight viruses and other pathogens. That's how it helps with genital warts caused by human papillomavirus (HPV), according to Mayo Clinic. Preliminary studies conducted in Hong Kong suggest that the cream may also enhance people's immune response to flu vaccines.
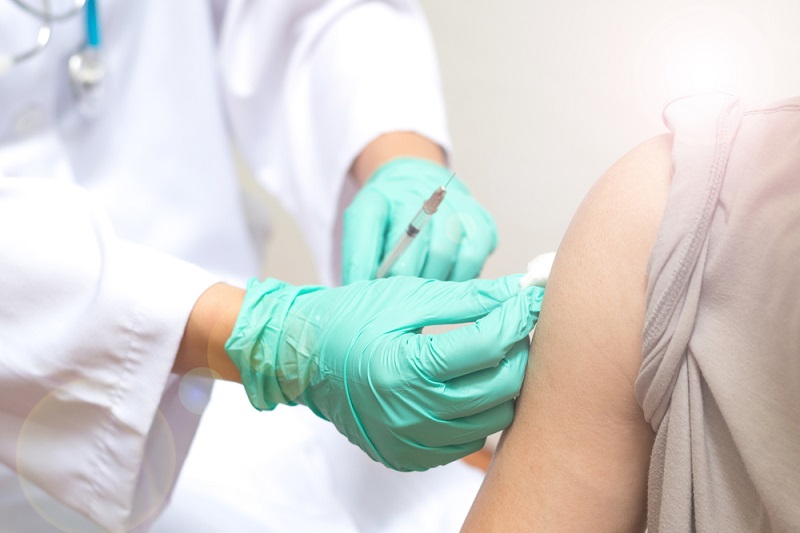
The new study aims to enroll 50 healthy adults who are 18 to 50 years old. Participants will be randomly assigned to receive either imiquimod or a placebo before their flu shot. The cream or placebo will be rubbed into participants' upper arms, and about 5 to 15 minutes later, they will receive a flu shot where the cream was applied. The vaccine will be delivered into the skin using a "microneedle injector," the NIH said.
Participants will then be tracked for seven months and have blood samples taken so that the researchers can evaluate their immune response to the vaccine. The study began in June and researchers hope to have early results by the end of the year.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Original article on Live Science.

Rachael is a Live Science contributor, and was a former channel editor and senior writer for Live Science between 2010 and 2022. She has a master's degree in journalism from New York University's Science, Health and Environmental Reporting Program. She also holds a B.S. in molecular biology and an M.S. in biology from the University of California, San Diego. Her work has appeared in Scienceline, The Washington Post and Scientific American.
 Live Science Plus
Live Science Plus





