The Chemistry of Life: Where Oil Comes From
Editor's Note: This occasional series of articles looks at the vital things in our lives and the chemistry they are made of.
Oil, the lifeblood of U.S. transportation today, is thought to start with the remnants of tiny organisms that lived millions of years ago, but the exact chemical transformation is somewhat mysterious. New research is looking at the role played by microorganisms that live in the deep dark bowels of the Earth.
A minority of scientists say otherwise, but most geologists think that the petroleum we pump from the ground (and later refine into gasoline and other fuels) comes predominantly from the fossils of marine life, such as algae and plankton.
"There is a lot of evidence to support the biogenic origin," said Everett Shock, a biogeochemist at Arizona State University. "Some of the petroleum molecules, for example, resemble the lipids found in bacterial cell membranes."
Whereas most of the dead material in the ocean is recycled by bacteria, lipids are tough, fat-like molecules that "tend to be the least desirable to eat," Shock said. They generally get passed up and fall to the seafloor, where they become buried under layers of sediment and eventually cooked into petroleum.
Once the organic remains become entombed in rock, most scientists have assumed that biology ends and geology takes over. However, deep drilling expeditions in the past few decades have discovered bacteria living thousands of feet below the surface, at the same depths where petroleum is forming.
"Are these microorganisms directly involved in the reactions that turn organic material into petroleum?" asked Shock.
Get the world’s most fascinating discoveries delivered straight to your inbox.
He is leading a research group funded by the National Science Foundation that aims to figure out what these deep-dwelling microbes may be living off of and what influence they may have on petroleum chemistry.
Oil battery
Even if some uncertainty remains over the exact chemical pathway to oil, the starting point is not in doubt.
"The ultimate source of energy is the sun, and oil is just a 'battery,'" said Barry Katz, a research scientist at Chevron.
Plants and certain bacteria use sunlight to convert carbon dioxide into sugar. This stored chemical energy is passed along the food chain, and a few "crumbs" wind up getting buried underground.
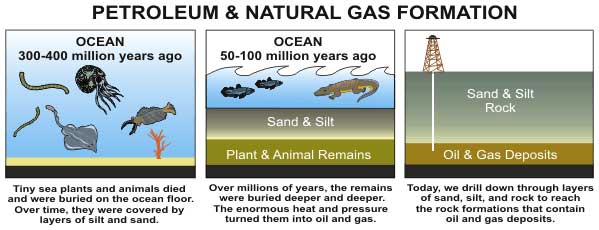
Once there, this organic material is transformed by heat and pressure into a complex mixture called kerogen. Depending on the initial ingredients and the geologic conditions, kerogen can produce either coal (a solid carbon-rich fuel derived mostly from woody plants) or hydrocarbons (a relatively hydrogen-rich substance that comes from algae and various lipid-containing plant parts).
Hydrocarbons are typically long chains of carbon and hydrogen atoms. The smaller hydrocarbon molecules (such as methane, propane and butane) are found in natural gas. The larger hydrocarbons (such as hexane and octane) make up petroleum.
As was mentioned, certain types of kerogen will form and release hydrocarbons — typically when the temperature rises above 212 degrees Fahrenheit (100 degrees Celsius).
"It's a very inefficient process," Katz said. "Less than 1 percent of the organic material growing in the ocean becomes hydrocarbons."
Even when oil does form, it does not always last. Some of it migrates up to the surface, where oil-eating microbes consume the better parts of it (creating so-called tar sands). To prevent this from happening, there needs to be a geologic formation that can trap the petroleum in a reservoir.
"Charging" this oil battery can take anywhere from 1 million to 1 billion years, with most petroleum we use being around 100 million years old.
Energy drain
The chemically stored solar energy is whittled away by the long and intricate process of petroleum formation.
"Petroleum in the ground is at a low energy state," Shock told LiveScience. "It only becomes energetic when we bring it up to the surface and introduce it to an oxygen atmosphere."
The reduced energy potential of buried organic material begs the question: what are deep-dwelling microbes surviving on?
"We don't know what they do," Shock said. "We just met them."
One possibility is that they are eating small organic byproducts that get expelled from the kerogen at the same time as the hydrocarbons. The other possibility is that these hearty bugs are actively helping to catalyze the reactions that create oil and siphoning off a bit of the remaining energy for themselves.
Simulating at high speed
Shock's team plans to create petroleum in the lab to see if there is any aspect of the process that might support bacteria.
This won't be the first time that scientists have simulated natural petroleum formation. To speed up the cooking process, researchers generally turn the temperature up to several 100 degrees Celsius.
"No one wants to wait around 10 million years for an experiment to finish," Shock said.
The assumption is that the same reactions occur at both high and low temperatures, but no one can say for sure that this is the case.
"It's rather remarkable that we are so dependent on oil, and yet we really don't understand how it is made in all its gory details," Shock said.
Perhaps these subterranean microbes will help fill in the missing pieces.
- Video - The Truth About Solar Power
- Video - The Story of Wind Power
- Black Gold: Where the Oil Is
 Live Science Plus
Live Science Plus