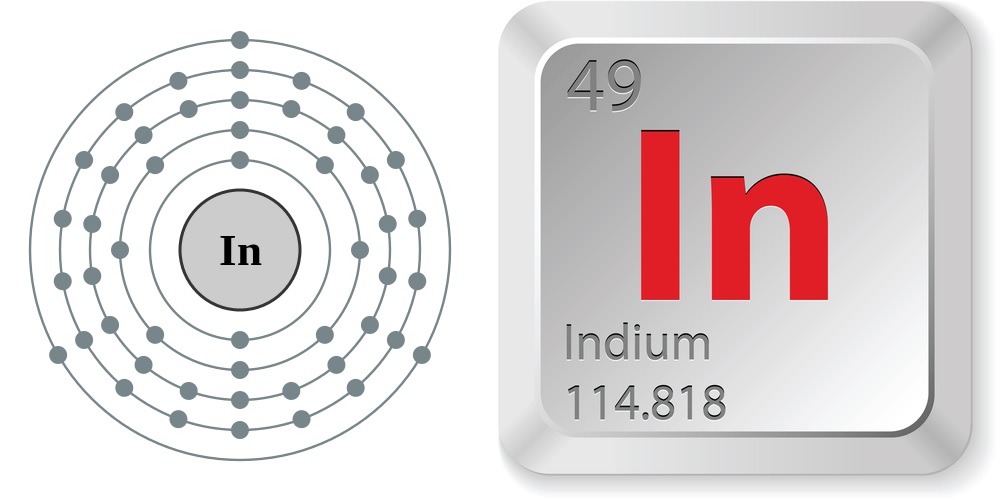
Facts About Indium

Indium is a lustrous silvery metal that is so soft and malleable it can be scratched with a fingernail and bent into nearly any shape. In nature, indium is quite rare and nearly always found as a trace element in other minerals — particularly in zinc and lead — from which it is typically obtained as a byproduct. Its estimated abundance in the Earth's crust is 0.1 parts per million (ppm) — a little more abundant than silver or mercury, according to the Royal Society of Chemistry.
Indium has a low melting point for a metal: 313.9 degrees Fahrenheit (156.6 degrees Celsius). At anything above this temperature, it burns with a violet or indigo flame. Indium's name is derived from the brilliant indigo light it shows in a spectroscope.
Just the facts
- Atomic number (number of protons in the nucleus): 49
- Atomic symbol (on the periodic table of the elements): In
- Atomic weight (average mass of the atom): 114.8.8
- Density: 7.31 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 313.88 degrees F (156.6 degrees C)
- Boiling point: 3,761.6 F (2,072 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 35 whose half-lives are known; 1 stable; 2 naturally occurring
- Most common isotope: In-115
Discovery
Indium was discovered in 1863 by the German chemist Ferdinand Reich at the Freiberg School of Mines in Germany. Reich was studying a sample of a zinc mineral blend that he thought might contain the recently discovered element thallium. After roasting the ore to remove most of the sulfur, he applied hydrochloric acid to the remaining materials. He then observed a yellowish solid appear. He suspected this could be the sulfide of a new element, but since he was color blind, he asked fellow German chemist Hieronymous T. Richter to examine the sample's spectrum. Richter noted a brilliant violet-colored line, which did not match the spectral line of any known element.
Working together, the two scientists isolated a sample of the new element and announced its discovery. They named the new element indium, after the Latin word indicum, meaning violet. Unfortunately, their relationship turned sour when Reich learned that Richter had claimed to be the discoverer, according to the Royal Society of Chemistry(RSC).
Uses
More than a century after indium's discovery, the element still lay in relative obscurity since no one knew what to do with it. Today, indium is vital to the world's economy in the form of indium tin oxide (ITO). This is because ITO remains the best material to fill the growing need for LCD's (liquid crystal displays) in touch screens, flat screen TVs and solar panels.
ITO has several properties that make it perfect for LCD's and other flat panel displays: It is transparent; conducts electricity; adheres strongly to glass; resists corrosion; and is chemically and mechanically stable.
ITO is also commonly used to make thin coatings for glass and mirrors. When coated over the windshields of aircraft or cars, for example, ITO allows the glass to de-ice or de-mist, and it can reduce air conditioning requirements.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The growing demand for LCDs has increased indium's prices considerably in recent years, according to the RSC. However, recycling and manufacturing efficiency have helped create a good balance between supply and demand.
Indium is commonly used to make alloys and is often referred to as the "metal vitamin," meaning that tiny levels of indium can make a drastic difference in an alloy, according to the RSC. For example, adding small amounts of indium to gold and platinum alloys makes them much harder. Indium alloys are used to coat the bearings of high-speed motors and other metal surfaces. Its low-melting alloys are also used in sprinkler heads, fire-door links, and fusible plugs.
Indium metal remains unusually soft and malleable at very low temperatures, making it perfect for use in tools needed in extremely cold conditions, such as cryogenic pumps and high vacuum systems. Another unique quality is its stickiness, making it very useful as a solder.
Indium is used in the making of various electrical devices such as rectifiers (devices that convert an alternating current into a direct one), thermistors (an electrical resistor dependent on temperature) and photoconductors (devices that increase their electrical conductivity when exposed to light).
Source & abundance
Indium is rarely found uncombined in nature and is typically found in zinc, iron, lead and copper ores. It is the 61st most common element in the Earth's crust and around three times more abundant than silver or mercury, according to the U.S. Geological Survey (USGS). It is estimated to make up around 0.1 parts per million (ppm) in the Earth's crust. By weight, indium is estimated to be 250 parts per billion (ppb), according to Chemicool. Natural indium is a mixture of the isotopes I-115 (95.72 percent) and I-113 (4.28 percent), according to Encyclopaedia Britannica.
Most commercial indium comes from Canada and is around 75 tons per year. Reserves of the metal are estimated to exceed 1,500 tons. Cultivated soils are sometimes found to be richer in indium than non-cultivated soils with some levels as high as 4 ppm, according to Lenntech.
Who knew?
- Indium metal gives off a high-pitched "scream," when bent. Similar to the "tin cry," this scream sounds more like a crackling sound.
- Indium is similar to gallium in that it readily wets glass and is very useful for making low-melting alloys. An alloy consisting of 24 percent indium and 76 percent gallium is liquid at room temperature.
- The first large-scale indium application was a coating for bearings in high-performance aircraft engines in World War II, according to the USGS.
- Specimens of uncombined indium metal has been found in a region of Russia, according to Lenntech.
Better batteries
Indium coating could one day lead to more powerful and longer-lasting rechargeable lithium batteries, according to a study published in the journal Angewandte Chemie. The indium coating would offer a more uniform depositing of lithium while charging, buffer any negative side-reactions and increase storage.
A lithium-ion battery is a type of rechargeable battery commonly used in portable technologies, such as cell phones and laptop computers. During discharge, the lithium ions move from the negative electrode (anode) to the positive electrode (cathode). While the battery is charging, the lithium ions travel in the opposite direction — the negative electrode becomes the cathode, and the positive electrode becomes the anode.
Currently, lithium-ion batteries use anodes made of graphite that are used to store lithium when the battery is charged. A promising alternative to using graphite would be metallic anodes — such as lithium metal — which could offer much greater storage capacity. However, a major problem with using metallic anodes is that there is an uneven deposition of the metal while the battery is charging. This leads to the formation of dendrites (a crystal mass with a branching tree-like structure). After prolonged use, these dendrites grow so large that they short-circuit the battery.
Another problem with metallic anodes is that they cause undesirable side-reactions between the reactive metal electrodes and the electrolyte (the material that allows electricity to flow between positive and negative electrodes). These reactions can significantly reduce the battery's life.
Researchers from Rensselaer Polytechnic Institute and Cornell University have introduced a new alternative: coating the lithium in an indium salt solution. The indium layer is uniform and self-healing when the electrode is in use. Its chemical composition remains the same, and it remains intact during charge/discharge cycles, preventing side-reactions, according to the study press release in Science Daily. Dendrites are also eliminated, allowing the surface to remain smooth and compact.