Facts About Titanium

Is there any element that calls to mind the notion of strength quite like titanium? Named after the Titans, Greek gods of myth, the 22nd element on the Periodic Table appears in airliners and lacrosse sticks, body piercings and medical equipment and even sunscreen.
Titanium resists corrosion and is particularly strong and lightweight. It's as strong as steel, but only 45 percent the weight, according to Los Alamos National Laboratory. And it's twice as strong as aluminum, but only 60 percent heavier.
Just the facts
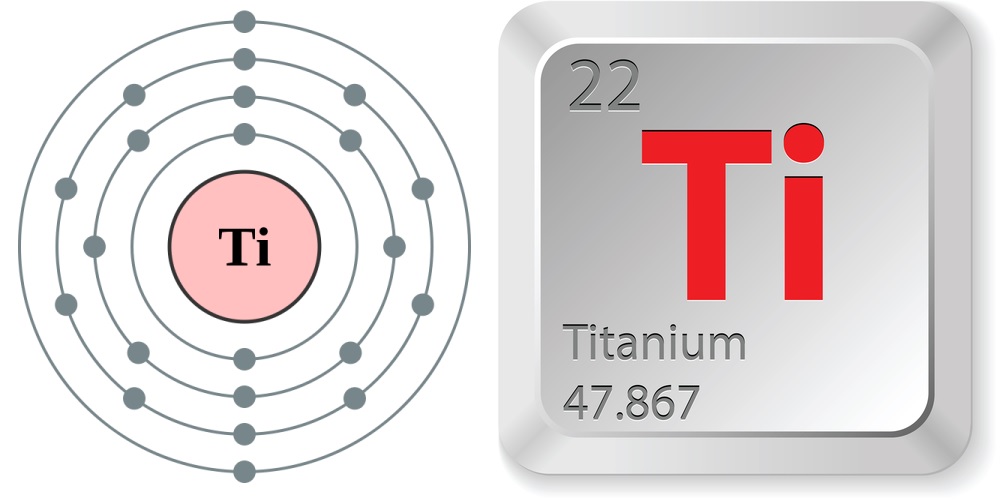
- Atomic number (number of protons in the nucleus): 22
- Atomic symbol (on the Periodic Table of Elements): Ti
- Atomic weight (average mass of the atom): 47.867
- Density: 4.5 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 3,034.4 degrees Fahrenheit (1,668 degrees Celsius)
- Boiling point: 5,948.6 F (3,287 C)
- Number of isotopes: 18; five are stable
- Most common isotopes: Titanium-46, Titanium-47, Titanium-48, Titanium-49 and Titanium-50
Superhero element
For an element with superpowers, titanium has a fitting origin story: It is forged in the depths of supernovas, or collapsing stars. A 2012 study of a particular dying star, Supernova 1987A, found that a single supernova can create 100 Earths worth of titanium-44, a radioactive isotope of titanium, by mass.
Titanium is the ninth most abundant metal in Earth's crust, according to Chemicool, but it wasn't discovered until 1791. English amateur geologist Rev. William Gregor discovered some black, metallic sand in a creek bed, analyzed it and discovered it to be a mixture of magnetite, a common form of iron oxide, and a new metal. Gregor called it manaccanite for the parish in which he'd discovered the sand.
Four years later, a German scientist named Martin Heinrich Klaproth was studying an ore from Hungary when he realized it contained a never-before-described chemical element. He called it titanium, and later confirmed that Gregor's manaccanite contained titanium, too.
The first person to distill titanium into its pure form was M.A. Hunter, an employee of General Electric, according to the Royal Society of Chemistry (RSC). It wasn't until the 1930s, however, that William J. Kroll invented a process that made extracting titanium possible on an industrial scale. The so-called Kroll process first treats titanium oxide ore with chlorine, producing titanium chloride. Next, magnesium or sodium are mixed with the titanium chloride in argon gas (allowing oxygen into the proceedings would be quite explosive, indeed, given that titanium is highly reactive to oxygen, according to the RSC). Under temperatures of 2,192 F (1,200 C), the magnesium or sodium reduces the titanium chloride to pure titanium. This process is about 10,000 times less efficient as the process used to make iron, according to the RSC, which helps explain why titanium is the more expensive metal.
Titanium is a transition metal, meaning it can form bonds using electrons from more than one of its shells, or energy levels. It shares this feature with other transition metals, including gold, copper and mercury.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Who knew?
- Almost every igneous rock — rocks formed from the solidification of molten rock — contains titanium, according to the RSC.
- A Boeing 737 Dreamliner is made of 15 percent titanium, according to the company.
- Titanium is orbiting the planet right now: The International Space Station (ISS) has a number of titanium parts, including pipes, according to NASA. The Rosetta Project, a research and archiving venture with the goal of preserving human languages and thought, has also flown an etched piece of pure titanium outside the ISS, to see how it stands up to radiation and the harsh environment of space.
- Earth isn't the only place to find titanium. In 2011, a satellite map of the moon's surface revealed clusters of titanium-rich rock. These rocks often contained up to 10 percent titanium, compared to the 1 percent or so typically seen in Earth rocks.
- Titanium can be used as a raw material in 3D printing. In 2013, researchers at Australia's Commonwealth Scientific and Industrial Research Organization 3D-printed a pair of lightweight titanium horseshoes for racehorses. The shoes were a stylish hot pink.
Titanium dioxide
Titanium dioxide (TiO2), also called titanium(IV) oxide or titania, is the naturally occurring oxide of titanium. A white pigment, titanium dioxide is used in paints (as titanium white or pigment white 6), and sunscreen because of its ability to refract light and absorb ultraviolet rays. According to the U.S. Geological Survey, 95 percent of titanium mined is turned into titanium dioxide pigments, with the remaining 5 percent going into manufacturing chemicals, metal, carbides and coatings.
Titanium dioxide is also commonly used in medicine, cosmetics and toothpaste, and is increasingly being used as a food additive (as E171) to whiten products or make them look more opaque. Some of the more common food products with added E171 include frosting, chewing gum, marshmallows and supplements.
There are no restrictions on the use of titanium dioxide in food products. However, a new study on mice, published in the journal Gut, shows that titanium dioxide particles may be very damaging to the intestines of those with certain inflammatory bowel diseases.
Researchers at the University of Zurich in Switzerland found that when intestinal cells absorb titanium dioxide particles, the intestinal mucosa of mice that had colitis became inflamed and damaged, according to the study news release.
Inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis have been on the rise in Western countries for many years. These conditions are characterized by an extreme autoimmune reaction to intestinal flora. Several factors play a role in the development of the disease, including genetics and environmental triggers such as lifestyle and nutrition. Now the Swiss researchers have found that titanium dioxide nanoparticles, commonly found in toothpaste and many food products, can exacerbate this inflammatory reaction to an even greater degree.
In addition, higher concentrations of titanium dioxide particles can be found in the blood of patients with ulcerative colitis. This means that these particles can be absorbed from food under certain disease conditions, explain the researchers in the news release.
Though the findings have not been confirmed in humans yet, the researchers suggest that patients with colitis should avoid ingesting titanium dioxide particles.
Current research
Titanium dioxide had a dizzying array of functions in the tech world, from solar cell applications to biocompatible sensors, said Jay Narayan, a materials scientist at North Carolina State University.
In 2012, Narayan and his colleagues reported a way to "tune" titanium dioxide, customizing it for particular applications. This material comes in two crystalline structures, called "rutile" and "anatase," each of which has its own properties and functions. Usually, titanium dioxide likes to be in the anatase phase below 932 F (500 C), and transforms to the rutile phase at hotter temperatures.
By growing titanium dioxide crystal-by-crystal and lining them up on a template made of titanium trioxide, Narayan and his colleagues were able to set the material's phase as either rutile or anatase at room temperature, they reported in June 2012 in the journal Applied Physics Letters. In an even bigger leap, the researchers were able to integrate this titanium dioxide into computer chips.
"Titanium oxide is also a very good sensor material, so if it is integrated with a computer chip then it acts like a smart sensor," Narayan told Live Science. As the sensor is part of the chip, the device can respond more rapidly and efficiently than if the sensor were separate and had to be hard-wired to the computing portion of the device.
Getting the product to market will require manufacturing costs to come down, Narayan said, but the "tunable" titanium dioxide has other promise, as well. By zapping the material with high-powered laser pulses, researchers can create small defects, called oxygen vacancies, where the material is missing oxygen molecules. The material can then be used to split water (H2O) by stealing the oxygen and leaving hydrogen, which can then be used to make hydrogen fuel.
"This is a cheap source of energy — and clean," Narayan said.New manufacturing and engineering methods are expanding titanium's uses. The Office of Naval Research announced in 2012 that a new method of welding titanium would be used to produce a full-sized ship hull; the construction is a breakthrough, according to the Navy, because titanium is typically too expensive and difficult to manufacture for shipbuilding. The new method, called friction stir welding, uses a rotating metal pin to partially melt the edges of two pieces of titanium together.
In medicine, titanium implants are used to replace or stabilize broken bone. Tiny titanium implants are even used to improve hearing in people with some types of deafness. A screw-like titanium rod is drilled into the skull behind the ear and attached to an external sound-processing unit. The external unit picks up sounds and transmits the vibration through the titanium implant to the inner ear, bypassing any problems in the middle ear.
In 2010, researchers announced the development of "Tifoam" a structure of polyurethane foam saturated with titanium powder. The porous structure mimics human bone and allows human bone cells to penetrate and meld with the implant as the person heals, according to a 2013 study on the material in the journal Acta Biomaterialia.
Additional reporting by Traci Pedersen, Live Science contributor.
Follow Stephanie Pappas on Twitter Google+. Follow us @livescience, Facebook & Google+.
Additional resources
- Learn more titanium facts from the Thomas Jefferson National Accelerator Facility (Jefferson Lab).
- The U.S. Geological Survey provides lots of statistics and information about titanium.
- Find more facts about titanium at the Royal Society of Chemistry.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.