Coronavirus news of the week (VIDEO)
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Since the discovery of the virus that causes COVID-19, the daily news cycle has become swamped with updates about how the pathogen spreads, what the bug does to the body and what solutions might finally bring an end to the pandemic.
But staying up-to-date on all the latest coronavirus news can be a challenge. To help keep you informed, we at Live Science have compiled a short list of standout news stories from the week — these are the ones that really caught our attention.
Related: 20 of the worst epidemics and pandemics in history
Steroids for critically ill patients
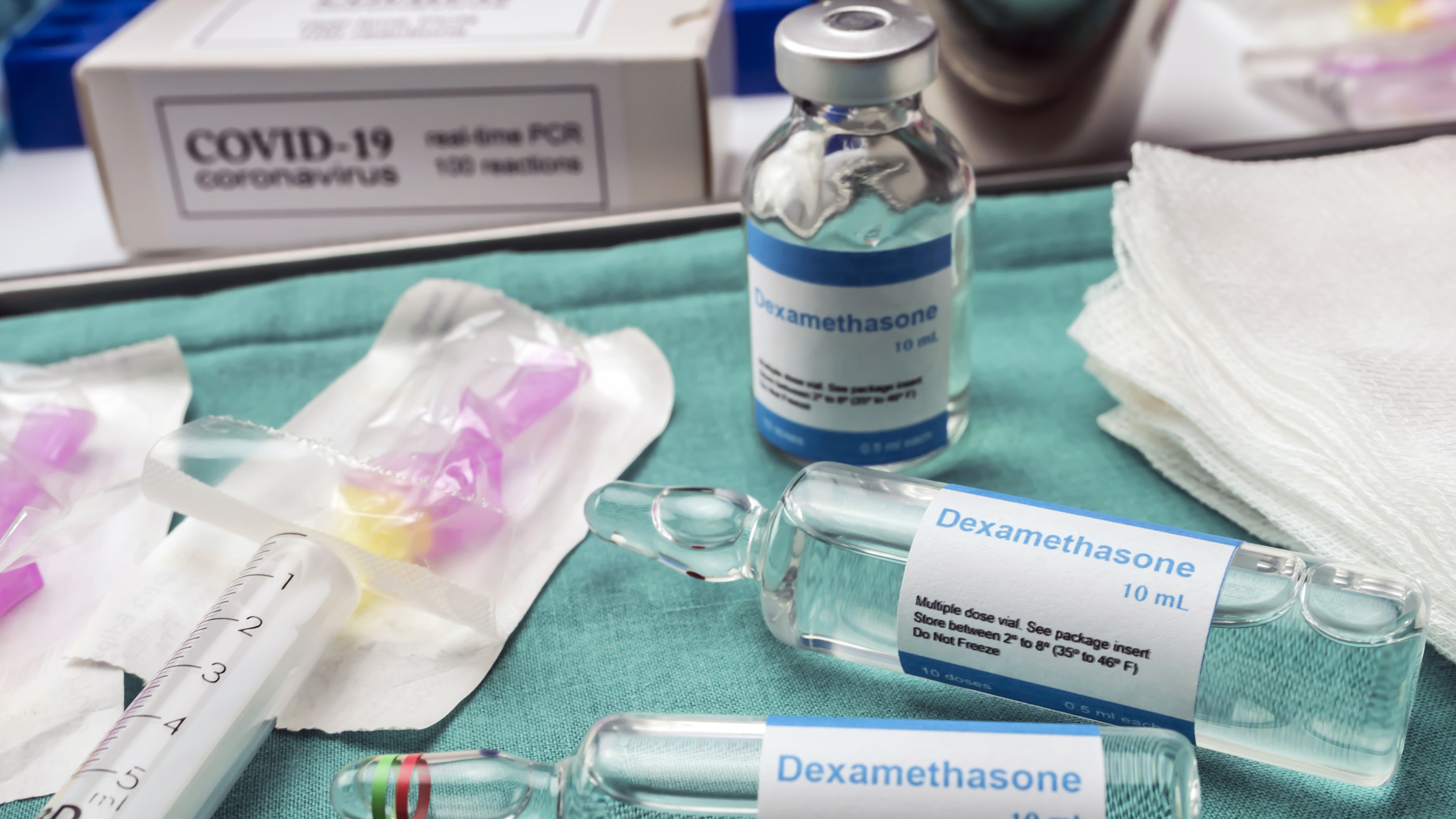
Inexpensive steroids can reduce the risk of death among critically ill COVID-19 patients, according to a new analysis published this week.
The analysis included data from seven randomized clinical trials, meaning that patients were randomly assigned to receive either corticosteroids or only standard care. Overall, the patients given steroids had a 34% reduced risk of death compared with those given standard care. The three steroids tested in the trials — dexamethasone, hydrocortisone and methylprednisolone — all work by suppressing the body's immune response and reducing inflammation. They also cause blood vessels to constrict and help to reduce fibrosis, or the development of thick scar tissue.
Based on these findings, the World Health Organization revised its treatment guidelines for corticosteroids and now recommends that the drugs be used as the standard of care for critically ill COVID-19 patients. Those with mild infections should not take steroids because "current data indicated they would not likely derive benefit and may derive harm" from taking the drugs, the guidelines state.
That viral statistic, explained

A statistic from the Centers for Disease Control and Prevention (CDC) fueled a bogus viral claim on social media this week, one that purports that the number of COVID-19 deaths in the U.S. is lower than officially stated. This claim is flat-out wrong.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The original CDC statistic stated that "only 6%" of people who have died of COVID-19 in the U.S. had no underlying conditions, such as diabetes, and didn't experience any medical complications during treatment, such as sepsis. The other 94% of people still died of COVID-19, but their prognoses was likely worsened by their existing medical conditions, experts told Live Science.
The false claim on social media misstated these stats to suggest that only 6% of the more than 180,000 COVID-19 deaths reported in the U.S. were actually caused by the virus; this is incorrect.
"The numbers you've been hearing — there are 180,000-plus deaths — are real deaths from COVID-19. Let there not be any confusion about that," Dr. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, said on "Good Morning America."
"The point is that those people [with underlying conditions] would not have died except that they were carried away by COVID," Dr. William Schaffner, a professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center in Tennessee, told Live Science. In other words, having underlying conditions makes you more likely to die from COVID-19.
Related: How are COVID-19 deaths counted?
Fast-tracked vaccines

COVID-19 vaccines could earn "emergency use authorization" before being thoroughly tested in advanced human trials, the head of the U.S. Food and Drug Administration (FDA) announced this week.
Normally, vaccines must pass through phase 3 clinical trials, which include tens of thousands of people, to earn FDA approval. Only these advanced trials can provide robust evidence that a vaccine is both safe and effective at preventing disease; but given the pressing need for a coronavirus vaccine, FDA Commissioner Dr. Stephen Hahn said that the agency may allow the emergency use of certain vaccine candidates before phase 3 trials are completed.
Emergency use authorization allows unapproved products to be used in an emergency to diagnose, treat or prevent serious or life-threatening conditions, "when there are no adequate, approved and available alternatives," according to the FDA. Hahn told The Financial Times that a vaccine cleared for emergency use would likely be authorized for only a select group of people.
Fauci argues that no vaccine should be authorized for emergency use without clear-cut evidence that it is both safe and effective. "One of the potential dangers if you prematurely let a vaccine out is that it would make it difficult, if not impossible, for the other vaccines to enroll people in their trial," Fauci previously told Reuters.
Originally published on Live Science.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus










