Facts About Fluorine

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
The most reactive element on the Periodic Table, fluorine has a violent history in the quest for its discovery. Despite the difficult and sometimes explosive properties of fluorine, it is a vital element for humans and animals, which is why it is commonly found in drinking water and toothpaste.
Just the facts
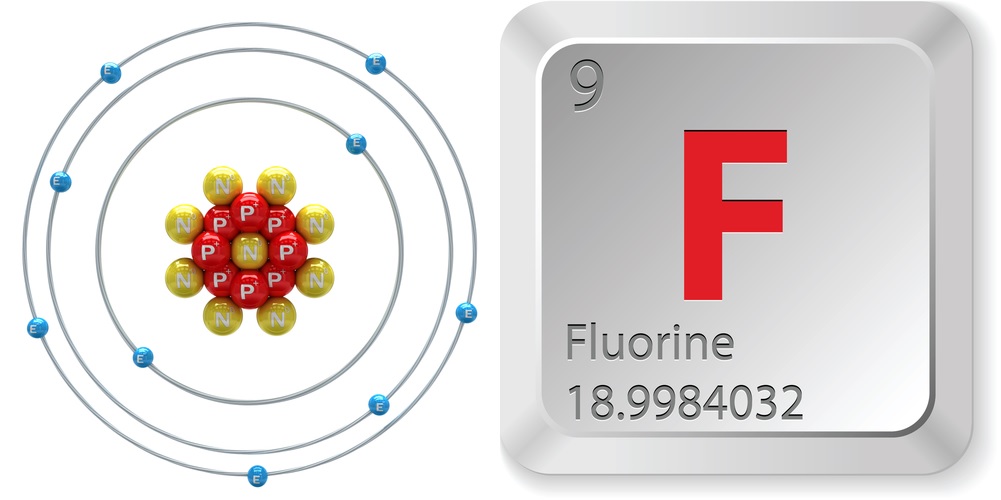
- Atomic number (number of protons in the nucleus): 9
- Atomic symbol (on the Periodic Table of Elements): F
- Atomic weight (average mass of the atom): 18.998
- Density: 0.001696 grams per cubic centimeter
- Phase at room temperature: Gas
- Melting point: minus 363.32 degrees Fahrenheit (minus 219.62 degrees Celsius)
- Boiling point: minus 306.62 degrees F (minus 188.12 degrees C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 18
- Most common isotopes: F-19 (100 percent natural abundance)
History
Early chemists tried for years to isolate the element from various fluorides. It wasn't until 1986 that German chemist Karl O. Christie successfully synthesized fluorine, and reported his results in the journal Inorganic Chemistry. Fluorine does not occur free in nature; but in 2012, researchers found small amounts of fluorine trapped in antozonite, a type of radioactive fluorite.
For centuries, the mineral fluorspar was used in metal refining. Known today as calcium fluoride (CaF2), it was used as a flux to separate pure metal from the unwanted minerals in ore, according to Chemicool. The "fluor" comes from the Latin word "fluere," meaning "to flow," because that's what fluorspar allowed metals to do. The mineral was also called Bohemian emerald and was used in glass etching, according to the Jefferson Laboratory.
Many scientists over the decades attempted to experiment with fluorspar to better learn its properties, as well as its composition. In their experiments, chemists often produced fluoric acid (today known as hydrofluoric acid, HF), an incredibly reactive and dangerous acid. Even small splashes of this acid on skin can be fatal, according to Chemicool. Several scientists were injured, blinded or killed in some of the experiments.
In the early 19th century, scientists Andre-Marie Ampere, in France, and Humphry Davy, in England, corresponded about the possibility of a new element within the acid. In 1813, Davy announced the discovery of the new element and named it fluorine from Ampere's suggestion.
Henri Moissan, a French chemist, finally isolated fluorine in 1886 — after being poisoned several times in his pursuit. He was awarded the Nobel Prize in 1906 for the isolating fluorine by electrolysis of dry potassium hydrogen fluoride (KHF2) and dry hydrofluoric acid.
Uses of fluorine
For many years, fluorine salts, or fluorides, have been used in welding and for frosting glass, according to the Royal Society. For example, hydrofluoric acid is used to etch the glass of light bulbs.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Fluorine is a vital element in the nuclear energy industry, according to the Royal Society. It is used to make uranium hexafluoride, which is needed to separate uranium isotopes. Sulfur hexafluoride is a gas used to insulate high-power electricity transformers.
Chlorofluorocarbons (CFCs) were once used in aerosols, refrigerators, air conditioners, foam food packaging, and fire extinguishers. Those uses have been banned since 1996 because they contribute to ozone depletion, according to the National Institutes of Health. Prior to 2009, CFCs were used in inhalers to control asthma but those types of inhalers were phased out in 2013.
Fluorine is used in many fluorochemicals, including solvents and high-temperature plastics, such as Teflon (poly(tetrafluoroethene), PTFE). Teflon is well known for its non-stick properties and is used in frying pans. It is also used for cable insulation, for plumber's tape and as the basis of Gore-Tex® (used in waterproof shoes and clothing).
Fluorine is added to city water supplies in the proportion of about one part per million to help prevent tooth decay, according to the Jefferson Lab. Several fluoride compounds are added to toothpaste, also to help prevent tooth decay.
Health and environmental impacts of fluorine
Although all humans and animals are exposed to and need minute amounts of fluorine, the element in any large enough dose is extremely toxic and dangerous. According to Lenntech, fluorine can naturally be found in water, air, and both plant and animal-based foods in small amounts. Larger amounts of fluorine are found in a few food products such as tea and shellfish.
While small amounts of fluorine are essential for maintaining the strength of our bones and teeth, too much can have the reverse effect of causing osteoporosis or tooth decay, as well has potentially harming the kidneys, nerves, and muscles.
In its gaseous form, fluorine is incredibly dangerous. Small amounts of fluorine gas can case eye and nose irritation while larger amounts can be fatal, according to Lenntech. Hydrofluoric acid, as another example, can also prove to be fatal when even a small splash on the skin occurs, according to Chemicool.
In the environment, fluorine, the 13th most abundant element in Earth's crust, typically settles within the soil and readily combines with soil, rock, coal and clay, according to Lenntech. Plants may absorb the fluorine from the soil, although high concentrations can lead to damage. Corn and apricots, for example, are among the plants that are most susceptible to damage and growth reduction when exposed to elevated levels of fluorine.
Who knew?
- Because fluorine is the most chemically reactive element, it must be handled with extreme care as it can sometimes explode on contact with all other elements excluding oxygen, helium, neon and krypton, according to Chemicool.
- Steel wool bursts into flames when exposed to fluorine, according to the Royal Society of Chemistry.
- Fluorine is also the most electronegative element. Fluorine attracts electrons more readily than any other element.
- On average, the amount of fluorine in the human body is three milligrams.
- Fluorine is primarily mined in China, Mongolia, Russia, Mexico and South Africa, according to Minerals Education Coalition.
- Fluorine is created in sun-like stars towards the end of their lifetime, according to a 2014 article published in the Astrophysical Journal Letters. The element is formed under the higher pressures and temperatures within the star when it expands to become a red giant. When the outer layers of the star are pushed away creating a planetary nebula, the fluorine travels along with the other gases into the interstellar medium eventually forming new stars and planets.
- According to the Journal of Chemistry, approximately 25 percent of drugs and medications, including those for cancer, the central nervous system, and the cardiovascular system, contain some form of fluorine.
Current research
Although fluorine can be toxic when the concentration within the body is too high, it can also be a beneficial element to include in cancer drugs, according to a 2018 article published in the Journal of Fluorine Chemistry. According to the research, replacing carbon-hydrogen or carbon-oxygen bonds with a carbon-fluorine bond in the active components of the drug usually shows an improvement of the drugs' effectiveness, including higher metabolic stability, increased binding to target molecules, and enhanced membrane permeability. It is hoped that with the increased effectiveness of the drugs, in conjunction with tumor-specific target drugs or targeted drug delivery systems, the quality of life of cancer patients can be greatly improved over traditional methods such as chemotherapy, in which cancer cells, as well as healthy cells, are targeted by the drugs.
This new generation of cancer-fighting drugs, as well as fluorine-probes to deliver the drugs, has been tested against cancer stem cells and has shown promise in targeting and fighting the cancer stem cells, according to the study. The researchers found that the drugs that included fluorine were several times more active against various cancer stem cells and exhibited better stability than traditional cancer-fighting drugs.
Additional resources
This article was updated on Nov. 24, 2020 to include information about the chemical synthesis of fluorine and the discovery of fluorine in nature.

Rachel Ross is a science writer and editor focusing on astronomy, Earth science, physical science and math. She holds a Bachelor of Arts in Philosophy from the University of California Davis and a Master's degree in astronomy from James Cook University. She also has a certificate in science writing from Stanford University. Prior to becoming a science writer, Rachel worked at the Las Cumbres Observatory in California, where she specialized in education and outreach, supplemented with science research and telescope operations. While studying for her undergraduate degree, Rachel also taught an introduction to astronomy lab and worked with a research astronomer.
 Live Science Plus
Live Science Plus