Scientists heat gold to 14 times its melting point — without turning it into a liquid
Scientists have used an ultrafast laser to heat solid gold to 14 times its melting point without turning the metal into liquid.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Scientists have used ultrafast high-intensity lasers to superheat gold to 14 times its melting point without turning the solid metal into a liquid.
The record-breaking experiment, which was described in a study published July 23 in the journal Nature, smashed a decades-old theory about the stability of solids and is the first reliable method to precisely measure the temperature of extremely hot systems, the researchers said.
Unusual states of matter, such as the plasma surrounding the sun or the high-pressure cores of planets, can reach incredible temperatures of millions of degrees Fahrenheit. However, actually putting a figure to this so-called "warm dense matter" has proven challenging, as scientists have struggled to measure the short-lived hot material fast enough to get reliable results.
"We have good techniques for measuring density and pressure of these systems, but not temperature," study co-lead author Bob Nagler, a scientist at the Department of Energy's SLAC National Accelerator Laboratory, said in a statement. "In these studies, the temperatures are always estimates with huge error bars, which really holds up our theoretical models — it's been a decades-long problem."
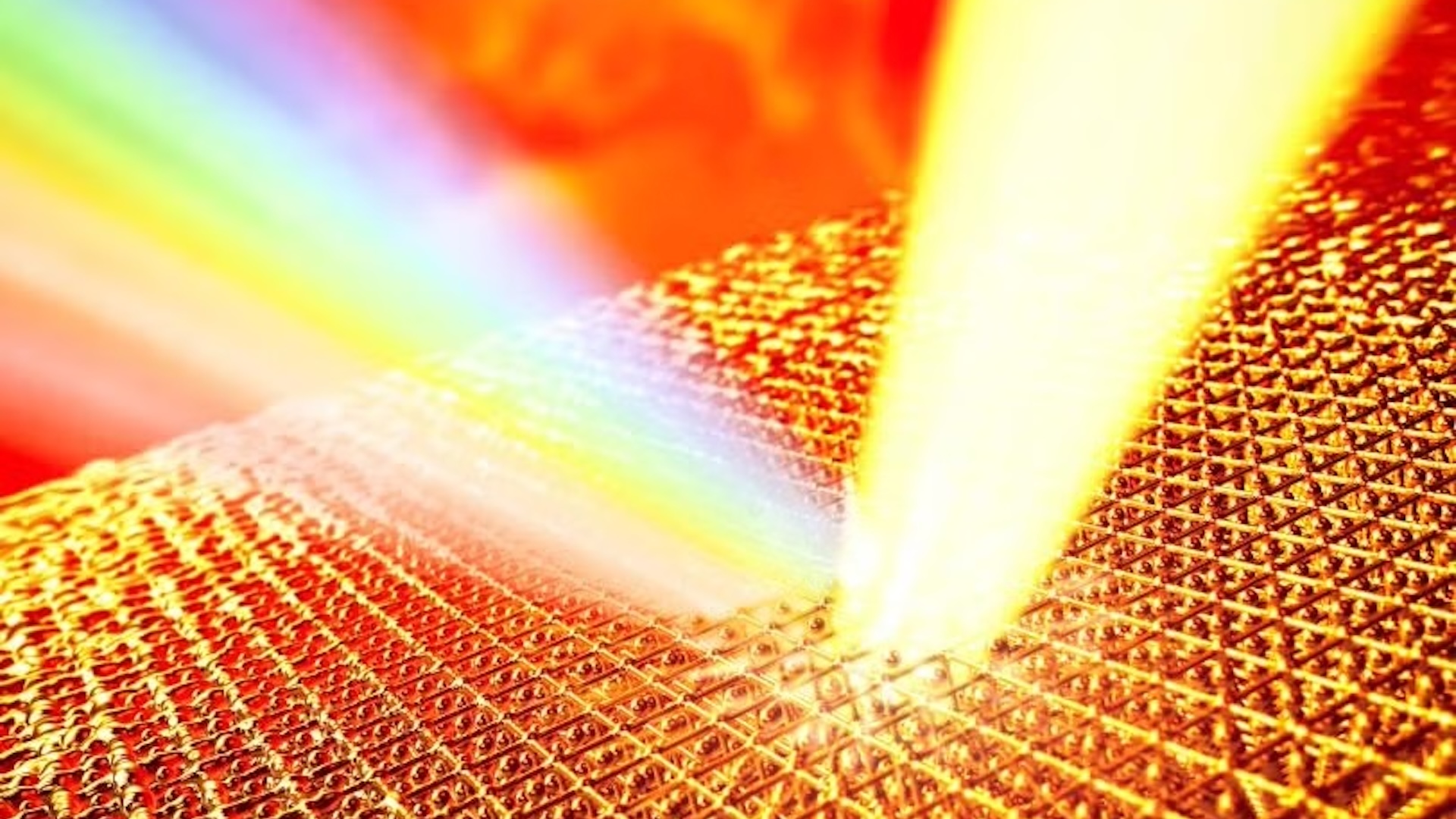
Speed was therefore key to taking a successful measurement. To achieve this, the team used 45-femtosecond (45 quadrillionths of a second) X-ray laser pulses to rapidly heat a thin gold film. As the radiation passed through the crystalline film, the atoms vibrated at a frequency directly related to their increasing temperature. A second pulse fired at the hot sample then scattered off these vibrating atoms, and the shift in frequency of these deflected beams provided a quantitative measurement of the atoms' speed and therefore temperature.
However, the researchers realized that they had achieved much more than a new measurement technique. "We were surprised to find a much higher temperature in these superheated solids than we initially expected, which disproves a long-standing theory from the 1980s," study co-lead author Thomas White, an associate professor of physics at the University of Nevada, Reno said in the statement.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The solid gold sample reached a staggering 19,000 kelvins (33,700 degrees Fahrenheit, or 18,700 degrees Celsius) — 14 times the element's standard melting point of 1,337 kelvins (1,947 F, or 1,064 C). "This is possibly the hottest crystalline material ever recorded," White added in another statement. "I was expecting the gold to heat quite significantly before melting, but I wasn't expecting a fourteen-fold temperature increase!"
Normally, solids and liquids have a defined temperature at which they change from one state to another. But under certain conditions, materials can be heated beyond these limits without changing state — a phenomenon known as superheating. This effect is sometimes seen in water heated in a microwave. If the container is smooth, there are no irregularities around which bubbles can form so the liquid water bypasses 212 F (100 C) without boiling. However, the slightest disturbance can trigger "catastrophe," and the water explosively boils as this metastable state is broken.
In the 1980s, physicists calculated the limit of this superheating effect for solids as three times the melting point, which they dubbed the "entropy catastrophe." Above this point, the solid would theoretically have a greater entropy, or disorder, than its liquid form, breaking the second law of thermodynamics. As this law states that entropy must always increase, the idea that the neatly arranged particles of a solid could be more disordered than the random distribution of particles in a liquid is an impossible contradiction.
So how did the gold sample remain solid at 14 times its melting point? The team suggested that the sheer speed at which they heated the gold prevented the crystal structure from expanding during the timescale of the experiment.
"It's important to clarify that we did not violate the second law of thermodynamics," White said. "What we demonstrated is that these catastrophes can be avoided if materials are heated extremely quickly — in our case, within trillionths of a second."
Periodic table of elements quiz: How many elements can you name in 10 minutes?

Victoria Atkinson is a freelance science journalist, specializing in chemistry and its interface with the natural and human-made worlds. Currently based in York (UK), she formerly worked as a science content developer at the University of Oxford, and later as a member of the Chemistry World editorial team. Since becoming a freelancer, Victoria has expanded her focus to explore topics from across the sciences and has also worked with Chemistry Review, Neon Squid Publishing and the Open University, amongst others. She has a DPhil in organic chemistry from the University of Oxford.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus





