Can other metals be turned into gold?
Other metals might be worth their weight in gold, but can they be turned into it?

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
In pursuit of prestige and riches, wealthy people across medieval Europe worked in vain to transmute everyday metals into gold. Today, this process, known as chrysopoeia, is mostly dismissed as an alchemical dream. But is there any science to show that metals can be turned into gold?
In fact, there is — but it would be far from a profitable business, evidence shows.
The idea of transmuting metals to gold goes back to ancient Greece and the philosopher Zosimos of Panopolis. He believed transforming lesser metals into gold was a reflection of the purification and redemption of the soul and the work had a deep spiritual significance. When the concept reemerged in medieval Europe, it was with a purely practical focus — converting a cheap metal into gold was a sure fire route to riches.
"Natural philosophers had this idea of ripening," Umberto Veronesi, an archaeologist and heritage scientist at the NOVA University Lisbon in Portugal, told Live Science. "Base metals were seen as impure stages and would eventually ripen to the purest form of all, which was gold. The only problem was that it would take a very long time for this to happen in the Earth."
Alchemists believed that if they could only create the philosopher's stone — a mythical substance — they would be able to catalyze this ripening process. Metals were thought to contain a mixture of fundamental ingredients: mercury, sulfur and salt. Therefore, by rearranging these components and drawing out any impurities, all metals would ultimately turn to gold, they hypothesized.

Sign up for our weekly Life's Little Mysteries newsletter to get the latest mysteries before they appear online.
"Chrysopoeia was generally consistent with theories of matter and theories of transformation at the time," Veronesi said. "Nobody really doubted that this could be done."
Related: Which is rarer: Gold or diamonds?
The emergence of modern science during the 17th and 18th centuries gradually discredited these ideas, and alchemy was abandoned in favor of the new disciplines of chemistry and physics. However, incredibly, nuclear scientists have held the secrets to this legendary transformation for almost a century.
Today, we know that the identity of an element is determined by the number of protons in its nucleus. Much-coveted gold atoms contain 79 protons, while lead has 82.
"The nucleus is held together by the strong force, and it's very difficult to remove a proton or neutron," said Alexander Kalweit, a physicist working at the Large Hadron Collider at CERN in Switzerland.
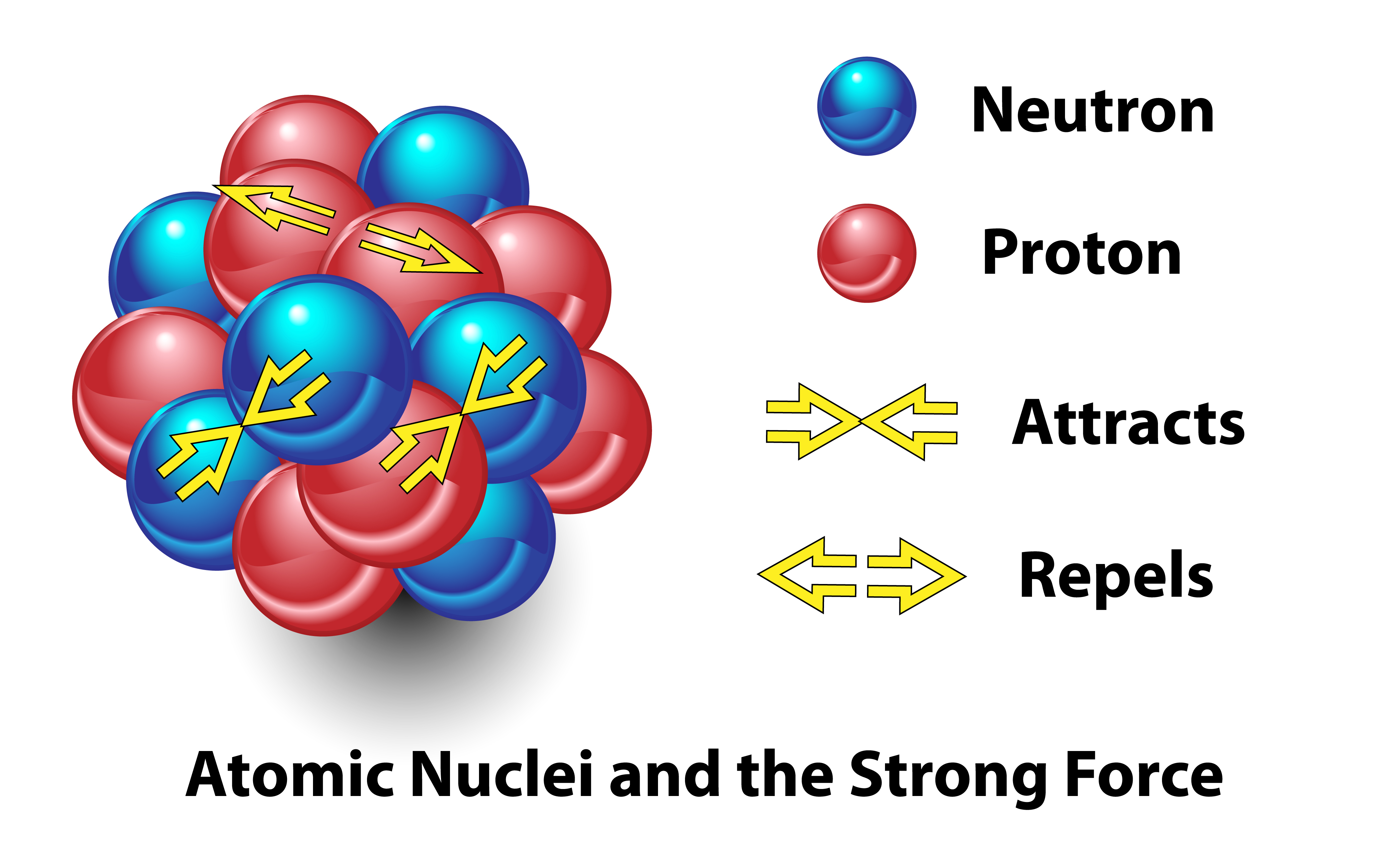
However, rearranging these fundamental components of an atom means it's theoretically possible to convert one element into another. "If you have enough energy, you can actually perform such operations," Kalweit said. "When you remove three protons from the lead nucleus, you have created a gold nucleus."
The first successful transmutation of another metal into gold was reported in 1941, when Harvard scientists used a particle accelerator to fire lithium and deuterium nuclei into atoms of mercury, which contains one proton more than gold does. The high-energy particles knocked protons and neutrons from the mercury nuclei, creating three short-lived radioactive isotopes of gold, which quickly decayed because the high-energy nuclei were unstable.
Forty years later, this extraordinary achievement was repeated by Nobel Prize in Chemistry winner Glenn Seaborg at Lawrence Berkeley National Laboratory in California. His team was investigating the fragmentation of bismuth nuclei in relativistic (speed-of-light) collisions and converted several thousand atoms of the element into gold by bombarding the sample with carbon and neon nuclei in a particle accelerator.
Today, research teams at particle accelerators around the world continue to report the production of gold as a by-product from their experiments. At the Large Hadron Collider, Kalweit's team is investigating the collisions of lead ions at close to the speed of light.
"In the head-on collisions, we essentially liberate the quarks that are inside the protons and neutrons, and they, for a short time, form a state of matter that existed a few microseconds after the Big Bang in the early universe," he explained. "It's the so-called quark gluon plasma."
These head-on collisions are so intense that the protons and neutrons are completely destroyed. But lower-energy near-miss interactions — where the particles are extremely close but not touching — generate a powerful electromagnetic field that knocks protons out of the lead nuclei. The result: The team detected around 29-trillionths of a gram of gold during a three-year experimental run.
However, despite having achieved the alchemist's dream, it's unlikely that nuclear physicists will ever turn a profit by synthesizing gold in a particle accelerator. The expense of building and running a facility like the Large Hadron Collider is astronomical compared with the value of the volume of gold produced; it's estimated that Seaborg's experiments in the 1980s cost around a trillion times the price of the gold they produced. Plus, the rarity of interesting interactions means researchers must wade through billions of data points to even identify the transformed atoms.
"Since the 1940s, there are many experiments which have produced gold," Kalweit said. "But what is common to all of them is that none of them is even remotely close to being profitable."
Periodic table of elements quiz: How many elements can you name in 10 minutes?

Victoria Atkinson is a freelance science journalist, specializing in chemistry and its interface with the natural and human-made worlds. Currently based in York (UK), she formerly worked as a science content developer at the University of Oxford, and later as a member of the Chemistry World editorial team. Since becoming a freelancer, Victoria has expanded her focus to explore topics from across the sciences and has also worked with Chemistry Review, Neon Squid Publishing and the Open University, amongst others. She has a DPhil in organic chemistry from the University of Oxford.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus











