Facts About Sulfur

Ick, what's that smell? If the stench is of rotten eggs, it might just be the fault of sulfur. This bright yellow element, known in the Bible as "brimstone," is abundant in nature, and was used for a variety of purposes in ancient times.
A nonmetal, sulfur is the 10th most abundant element in the universe, according to the Jefferson National Linear Accelerator Laboratory. Today, it's most common use is in the manufacture of sulfuric acid, which in turn goes into fertilizers, batteries and cleaners. It's also used to refine oil and in processing ores.
Pure sulfur has no smell. The stink associated with the element comes from many of its compounds, according to Chemicool. For example, sulfur compounds called mercaptans give skunks their defensive odor. Rotten eggs and stink bombs get their distinctive aroma because of hydrogen sulfide.
Just the facts
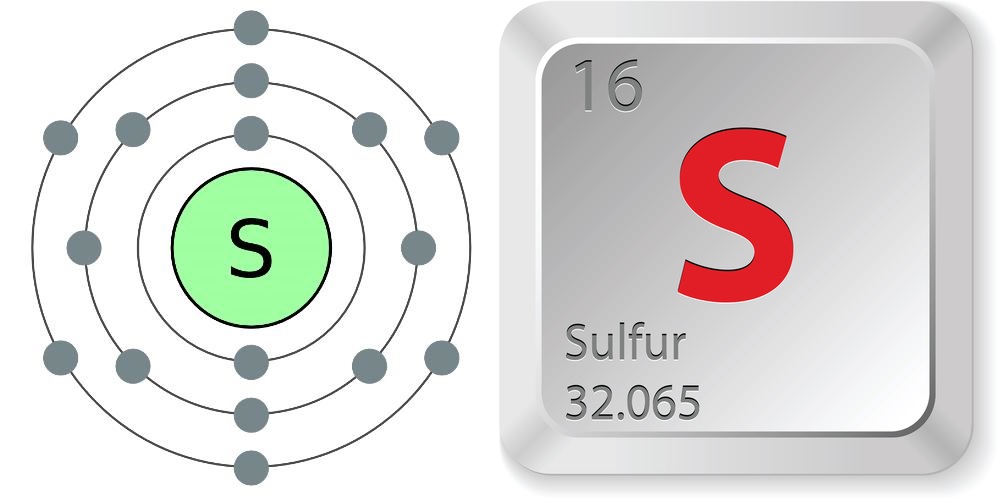
According to the Jefferson Lab, the properties of sulfur are:
- Atomic number (number of protons in the nucleus): 16
- Atomic symbol (on the Periodic Table of Elements): S
- Atomic weight (average mass of the atom): 32.065
- Density: 2.067 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 239.38 degrees Fahrenheit (115.21 degrees Celsius)
- Boiling point: 832.28 degrees F (444.6 degrees C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 23
- Most common isotopes: S-32 (94.99 percent natural abundance), S-33 (0.75 percent natural abundance), S-34 (4.25 percent natural abundance), S-36 (0.01 percent natural abundance)
An element of biblical proportions
"On the wicked he will rain fiery coals and burning sulfur; a scorching wind will be their lot." — Psalm 11:6
Few elements are high profile enough to get a mention in the Bible, much less 15 separate call-outs. But sulfur occurs frequently in compounds in nature, usually as a stinky, yellow mineral associated with hot springs and volcanoes, perhaps explaining why the authors of the Bible associated it with hellfire and wrath.
The element itself was not isolated until 1809, according to the Royal Society of Chemistry, when French chemists Louis-Josef Gay-Lussac and Louis-Jacques Thénard created a pure sample. (Gay-Lussac was known for his research on gases, which involved him flying in hydrogen-filled balloons more than 22,900 feet (7,000 meters) above sea level, according to the Chemical Heritage Foundation.)
When burned, sulfur produces a blue flame and sulfur dioxide gas — a common pollutant, according to the Environmental Protection Agency. Sulfur dioxide in the atmosphere comes mostly from fossil-fuel power plants and is one of the primary causes of acid rain. The gas is also a lung irritant. The EPA regulates sulfur dioxide emissions along with five other so-called "criteria pollutants," including lead and carbon monoxide.
Who knew?
- Sulfur makes up almost 3 percent of the Earth's mass, according to Chemicool. That is enough sulfur to make two additional moons.
- Sulfur (as sulfur dioxide) has been used to preserve wine for millennia, and remains an ingredient in wine today, according to the Practical Winery & Vineyard Journal.
- It's not clear where the name "sulfur" comes from. It could be derived from "sufra," or "yellow" in Arabic. Or it could be from the Sanskrit "shulbari," which means "enemy of copper." The second possibility is intriguing, according to Chemicool, because sulfur does react strongly with copper. Did ancient people know about this property of sulfur and name it accordingly?
- Sulfur dioxide was used to fumigate homes from ancient times, a practice which continued well into the 19th century. One 1889 paper by the New York City chief health inspector described how officials burned sulfur and alcohol in homes afflicted with smallpox, scarlet fever, diphtheria and measles.
- Ah, relaxation! Hot springs full of dissolved sulfur compounds may have a questionable smell, but they've long been prized for their supposed medicinal qualities. The town of Hot Sulphur Springs, Colorado, for example, sprang up in 1860 after white settlers discovered sulfur springs that Ute Indians had been soaking in for centuries.
- Wait, what's up with that spelling, anyway? "Sulphur" is the common spelling in the United Kingdom, while "sulfur" is preferred in America. But scientifically speaking, "sulfur" is correct, according to the International Union of Pure and Applied Chemistry, the organization whose job it is to determine these things. Thus, even British journals like Nature Chemistry use the "f" spelling.
- Sulfur can do a number on shipwrecks. A 2008 study of a Swedish warship that sank in 1628 found more than 2 tons of sulfur impregnating the timbers of the salvaged vessel.
- Excuse you! The main reason that intestinal gas has that nasty odor is that the large intestine is full of bacteria that release sulfur compounds as waste.
Current research
Today, sulfur is a byproduct of the refinement of fossil fuels into usable energy sources like gasoline. This refinement is a good thing for preventing sulfur compounds from heading skyward when the fuel is burned, causing acid rain. But it leads to hills of elemental sulfur piling up in refineries.
About 90 percent of this elemental sulfur goes to make sulfuric acid, said Jeff Pyun, a biochemist at the University of Arizona. But "since we go through millions of barrels of oil a day, a few percent [sulfur] a barrel just piles up quickly," Pyun said. With nearly 100 million tons of waste sulfur produced a year, the 10 percent not used in sulfuric acid production comes out to a not-insignificant 10 million tons a year.
What to do with this yellow mess? Pyun and his colleagues think they have an answer. They've found a way to turn waste sulfur into plastic, which in turn can be used in thermal imaging devices and lithium-sulfur batteries.
"It was a tremendous challenge, and we were the first crazy people to get really serious about it," Pyun told Live Science.
Sulfur is tough to work with because it doesn't dissolve in other chemicals easily. That was the first frustration Pyun and his team of researchers from Korea, Germany and the United States had to face.
"It didn't want to dissolve," Pyun said. "It just made yellow stuff everywhere, all over my lab."
At the end of their ropes, the researchers decided just to melt the stuff. It turns out that sulfur becomes a polymer — a long chain of linked molecules that is the basis for plastics — automatically when heated above 320 F (160 C). That reaction has been known for more than a century, Pyun said. But the polymer falls apart almost as easily as it forms, making it useless for practical applications.
But this polymer phase gave the researchers a window to "throw in something, potentially, that it would react with" to stabilize the plastic, Pyun said. Fortunately for the team, one of the first chemicals they tried turned out to be a winner: 1 3-diisopropylbenzene, easier known as "DIB."
"DIB works so nicely because it had reactive groups that could react with sulfur when it was polymerizing," Pyun said. "It was completely soluble in liquid sulfur."
The result, as the researchers reported in April in the journal Nature Chemistry, was a red plastic that doesn't even smell like rotten eggs — the polymerizing sulfur is not volatile, Pyun said, and thus doesn't reek like the volatile sulfur compounds one might find at a hot springs.
Even better, the process is so simple that Pyun and his colleagues call it "cave man chemistry." The simplicity and low cost make it an attractive option for industry, Pyun said. The team has been approached by several companies interested in taking the sulfur polymerization process commercial.
Which could be good news for the environment. Conventional oil and gas reservoirs are about 1 to 5 percent sulfur, Pyun said. More and more, however, oil and gas exploration is tapping into unconventional reservoirs filled with nastier stuff: The oil from tar sands in Alberta, Canada, is 20 percent sulfur. Some new fields in the Middle East produce oil that is up to 40 percent sulfur, Pyun added.
"We're only going to produce more sulfur," he said, adding that they refer to sulfur as the “garbage of transportation” because it is the byproduct of petroleum refining. With any luck, his team's process can turn that garbage into something useful.
Sulfur-based pesticide
Elemental sulfur is a commonly used pesticide on many American and European farms. It is approved for use on both conventional and organic crops to help control fungus and other pests. In California alone, more than 21 million kilograms (46.2 million pounds) of elemental sulfur was used for agriculture in 2013, according to Berkeley News.
Although the Environmental Protection Agency (EPA) has labeled elemental sulfur as generally safe, studies have shown that this type of pesticide is a respiratory irritant to farmworkers.
Now, a new study by researchers at the University of California, Berkeley, has gone a step further and looked at the respiratory health of residents living near treated fields — specifically, hundreds of children living in the agricultural community of Salinas Valley, California. Their findings were published in August 2017 in the journal Environmental Health Perspectives.
The researchers found that children living within a half-mile from recent elemental sulfur applications had reduced lung function, higher levels of asthma-related symptoms and greater asthma medication use, compared to unexposed children.
Specifically, they found that a 10-fold increase in applied sulfur within 1 kilometer (0.62 miles) of the child's residence during the year before the respiratory evaluation was associated with a 3.5-fold increased risk for asthma medication use and double the risk for respiratory symptoms, such as wheezing and shortness of breath, according to Berkeley News.
The study authors urgently call for more research to confirm these findings in the hope that it will lead to changes in regulations and application methods to limit respiratory harm on nearby residents. According to the researchers, one idea is to switch to "wettable" powders.
Additional reporting by Traci Pedersen, Live Science contributor. Follow Live Science @livescience, Facebook & Google+.
Additional resources
- See what the Jefferson Lab has to say about sulfur.
- Los Alamos National Laboratory also has detailed information about sulfur.
- The Royal Society of Chemistry provides facts about Element No. 16 as well.
Get the world’s most fascinating discoveries delivered straight to your inbox.

Stephanie Pappas is a contributing writer for Live Science, covering topics ranging from geoscience to archaeology to the human brain and behavior. She was previously a senior writer for Live Science but is now a freelancer based in Denver, Colorado, and regularly contributes to Scientific American and The Monitor, the monthly magazine of the American Psychological Association. Stephanie received a bachelor's degree in psychology from the University of South Carolina and a graduate certificate in science communication from the University of California, Santa Cruz.
