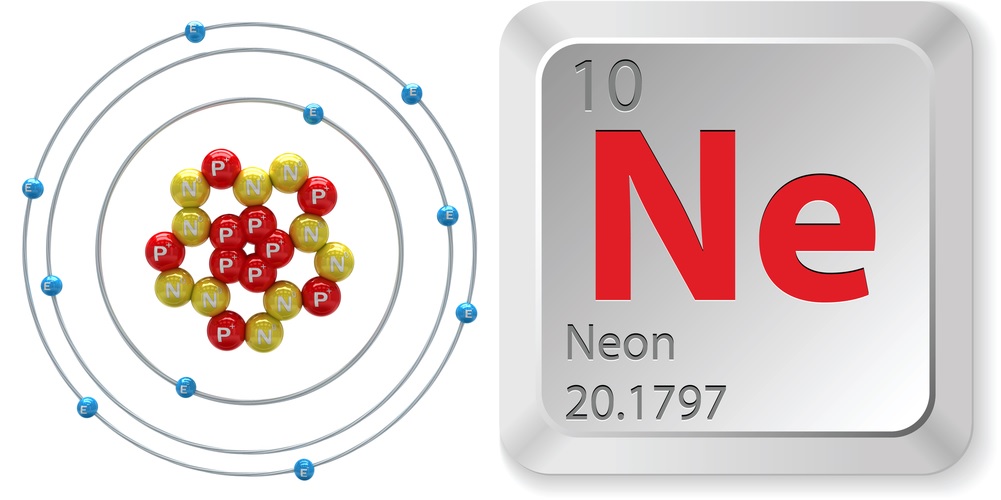
Facts About Neon

The gas that makes Las Vegas shine is one of the nobles — the noble gases, that is. Neon is one of six elements, found in the rightmost column of the Periodic Table, that are inert. Noble gases react very unwillingly, because the outermost shell of electrons orbiting the nucleus is full, giving these gases no incentive to swap electrons with other elements. As a result, there are very few compounds made with noble gases.
Like its noble gas comrades, neon is odorless and colorless. Under certain laboratory conditions, neon can form a compound with fluorine, but is otherwise nonreactive, according to the Thomas Jefferson National Accelerator Laboratory.
Just the facts
- Atomic number (number of protons in the nucleus): 10
- Atomic symbol (on the Periodic Table of Elements): Ne
- Atomic weight (average mass of the atom): 20.1797
- Density: 0.0008999 grams per cubic centimeter
- Phase at room temperature: Gas
- Melting point: minus 415.46 degrees Fahrenheit (minus 248.59 degrees Celsius)
- Boiling point: minus 410.94 degrees F (minus 246.08 degrees C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 19
- Most common isotopes: Ne-20 (90.48 percent natural abundance), Ne-22 (9.25 percent natural abundance), Ne-21 (0.27 percent natural abundance)
History
Chemists William Ramsay of Scotland and Morris Travers of England discovered neon in 1898, according to Chemicool. Ramsay had previously discovered argon in 1894 and was the first to isolate helium in 1895. From those elements' places on the Periodic Table, he deduced that there was a yet unknown element between the two noble gases.
Ramsay and Travers eventually discovered neon, as well as krypton and xenon, in an argon sample. The chemists froze the argon using liquid air, and then evaporated the argon and collected the gas that came off. They ran a high voltage through the first sample of the gas they had collected and surprisingly found that the tube with the glass glowed a bright crimson, according to Chemicool. Ramsay named the newfound element neon based on the Greek word neos, which means new.
Who knew?
- Neon is the fourth most abundant element in the universe, according to the Jefferson Laboratory.
- In Earth's atmosphere, neon only composes about 0.0018 percent, according to Chemicool.
- Neon is created in large mass stars when the internal pressure of the star is great enough to fuse carbon atoms into neon atoms, according to Berkeley Lab.
- According to Minerals Education Coalition, neon is primarily obtained from liquefying air.
- Neon has the smallest temperature range (2.6 degrees C or 4.7 degrees F) for which it is a liquid, according to Chemicool.
- Neon, along with helium, argon, krypton and xenon, make up the group known as noble gases. These are the most stable and least reactive elements due to having full valence shells (the outer shell has the max number of electrons, two for helium, eight for the rest). All noble gases conduct electricity, light up when a current runs through them, and are odorless, colorless and monatomic (exist as individual atoms).
- Due to its relative inertness, neon does not form any known stable compounds in nature, according to Chemicool.
- There are several researchers, with articles published in Nature and Foundations of Chemistry, who want to rearrange the Periodic Table to move helium next to hydrogen and promote neon to be the lightest of the noble gases due to properties of helium and the number of electrons in its outer shell.
- While generally inert and nontoxic, neon is also known as a simple asphyxiant, according to Lenntech. When inhaled, it may cause dizziness, nausea, vomiting and loss of consciousness. Death may be caused by errors in judgment, confusion, or being unconscious.
- According to the Jet Propulsion Laboratory, neon has been discovered in the planet-forming disks around young stars. The discovery of the neon in these disks helps astronomers track the gas content around the young stars during different stages of their development in order to better understand how planets form.
- When liquefied, neon is an important cryogenic refrigerant that has more than 40 times the refrigerating capacity per volume than liquid helium and more than three times that of liquid hydrogen, according to the Royal Society of Chemistry.
How a neon sign works
The most common use of the gas is in neon signs, which have a century-long history. First developed by French engineer Georges Claude in 1902, neon lights create light by applying electricity to neon or argon in a sealed glass tube. Argon makes blue light, and neon makes the clear orange-red that is familiar in neon signs, said Bill Concannon, a neon-sign artist and owner of Aargon Neon, a sign shop in Crockett, California.
Other colors are created by using a variety of other gases, such as argon, mercury, helium, krypton and xenon, according to the Edison Tech Center. Today, the majority of the lights you see in many places, such as on the Las Vegas strip, are made from mercury and argon and colored with phosphors.
Neon lights, also known as cold cathode fluorescent lamps (CCFL), work when electrodes on each end of a vacuum tube filled with neon or other fluorescing gas are subjected to an alternating current, according to the Edison Tech Center. The current ionizes the atoms causing the tube to be filled with free electrons. As the ionized atoms recapture their electrons to become neutral, visible light is released which gives the CCFL signs their colored glow.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Current research
Neon ratios in stars
Astronomers are studying the neon ratios in the sun to better understand not only our own star but also other stars in our universe. Two 2018 studies (Young and Brooks, et al.) found on arXivdiscuss the importance of the neon ratios. According to Young, the ratio of magnesium to neon is important for better understanding the ionization potentials in the solar atmosphere while the ratio of oxygen to neon could potentially aid in determining the amount of neon in the photosphere of the sun. And knowing these ratios, according to Brooks, et al., can help astronomers better understand the evolution of stars and the potentially the solar cycles of the star at the center of our solar system.
According to a press release article from the Massachusetts Institute of Technology, neon — along with carbon, oxygen, and nitrogen — is vital to the speed at which energy flows from the nuclear fusion reactions within the sun's core to its surface. The rate at which the energy flows directly relates to the location and size of the sun's convection zone.
Many elements, such as oxygen, carbon, and nitrogen, can be directly studied in the sun due to their spectral absorption lines. Neon, however, doesn't provide any usable spectral lines in the visible range of light, so the abundances of the element are deduced based on the ratios with others. The amounts of these elements within the sun are based on measurements taken from the photosphere, such as with the observation method from Young, or from the corona during eclipses according to the 2005 article from the press release.
Helium-neon lasers
Neon is used to make helium-neon lasers, which are relatively inexpensive. An electric charge excites the neon, and the neon atoms give off light as they return to their neutral state, similar to the way neon signs work, according to the University of California, Santa Barbara. Mirrors concentrate the light into a laser beam. Early LaserDisc players used helium-neon lasers to read the discs.
Protecting water supplies
In September 2014, researchers reported that drinking wells in Pennsylvania and Texas had been contaminated with methane as a result of badly sealed wells, rather than by the controversial practice of hydraulic fracturing, commonly dubbed "fracking," in which rock is fractured to release oil and natural gas. In the journal Proceedings of the National Academy of Sciences, the team of geochemists said they attached noble gases, such as neon and argon, to the methane in natural gas to trace the rogue methane because neon and argon don't react and thus move, unchanged, along with the natural gas.
Additional reporting by Stephanie Pappas, Live Science contributor.

Rachel Ross is a science writer and editor focusing on astronomy, Earth science, physical science and math. She holds a Bachelor of Arts in Philosophy from the University of California Davis and a Master's degree in astronomy from James Cook University. She also has a certificate in science writing from Stanford University. Prior to becoming a science writer, Rachel worked at the Las Cumbres Observatory in California, where she specialized in education and outreach, supplemented with science research and telescope operations. While studying for her undergraduate degree, Rachel also taught an introduction to astronomy lab and worked with a research astronomer.