For the first time, physicists peer inside the nucleus of a molecule using electrons as a probe
A novel experiment has revealed a phenomenon called the Bohr–Weisskopf effect in a pear-shaped nucleus in a molecule for the first time.

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Physicists have studied a rare molecule to look at how magnetism is distributed within a radioactive nucleus for the first time.
The rules of nature don’t, generally speaking, change. If you toss a ball in Seattle or in Tokyo, it falls the same way. Physicists call this “symmetry”, and they use symmetry as a guide to how the universe ought to behave. It’s what keeps the world consistent — if the laws of physics worked differently on Tuesdays, the universe would be chaos.
But some parts of nature don’t seem to follow this perfect balance. For example, it may seem fair to assume that the universe should treat matter and antimatter as equals. Yet our universe is made almost entirely of matter, and physicists still don’t know why.
One promising place to search for answers is inside radioactive nuclei. That is because the uneven arrangement of protons and neutrons can magnify the tiniest breaks in symmetry. If scientists are able to detect those small asymmetries, it could reveal new physics beyond the Standard Model, according to Silviu-Marian Udrescu, a physicist at MIT and co-author of a new study into the phenomenon.
In a study published Oct. 23 in the journal Science, scientists at CERN and MIT examined a short-lived radioactive molecule called radium monofluoride (RaF) to measure its energy spectrum. But, surprisingly, they ended up making the first observation of how magnetism is distributed within one of its nuclei. That phenomenon, known as the Bohr–Weisskopf effect, had never been seen in a molecule before.
The avocado of the atom
The RaF molecule is made of two atoms: radium and fluoride. Each with its own nucleus. The radium nucleus has a property called “octupole deformation”.
“You can think of it as the nucleus itself having the shape of a pear or an avocado,” Shane Wilkins, a physicist at MIT and the study’s first author, told Live Science. Because of its asymmetric shape, RaF makes a perfect candidate to find the asymmetries the team was looking for.
Get the world’s most fascinating discoveries delivered straight to your inbox.
“It’s a very rare property,” Udrescu added. “It only occurs in a few handfuls of atomic nuclei across the entire nuclear chart. And all of those nuclei that have this pear shape are radioactive.”
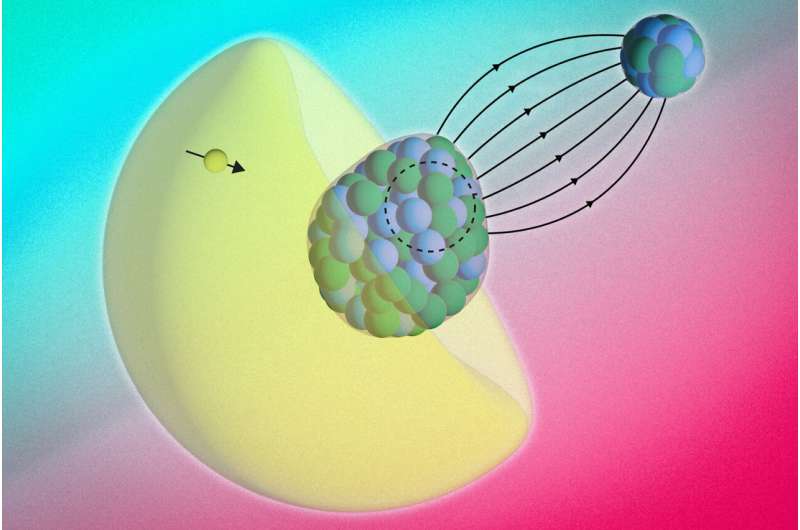
That radioactivity makes such nuclei difficult to study because these isotopes are unstable and short-lived. That means they decay within around 15 days, and can disappear before researchers can make many measurements. “We can only produce them in very small quantities,” Wilkins said.
The Bohr-Weisskopf effect has been observed in individual atoms, where electrons interact with a single nucleus. However, detecting it inside a molecule is more challenging. That is because electrons constantly move between the two nuclei. The movement can blur magnetic signals and make them harder to detect. In a RaF molecule, the fluoride atom is a simpler bond partner. It allows scientists to focus on the magnetic structure of the heavier radium nucleus.
The team first created radium monofluoride at CERN’s ISOLDE facility. They blasted a uranium target with high-energy protons to produce the rare isotope radium-225 and combined it with fluorine gas. Each molecule existed for only fractions of a second. The researchers could detect only about fifty per second in the right state for measurement.
Then, they directed multiple laser beams of slightly different frequencies at the molecules. When the molecule absorbed or emitted light, scientists recorded the tiny changes in that light. This produced a spectrum. Normally, those patterns tell scientists about how the electrons move around the nucleus. But in this case, some of the shifts revealed that the electrons were being influenced by the inside of the nucleus.
“The electron actually probes inside the nucleus, so you can no longer treat it as a long-range interaction. Instead, it starts to sense the internal properties of the radium nucleus itself,” said Wilkins.
“This effect is called the Bohr–Weisskopf effect,” Wilkins added. “To the best of our knowledge, it’s never been seen in a molecule before. The fact that we could both observe this effect experimentally and describe it with theory tells us a lot about how suitable these molecules are for future precision measurements.”
Now that the researchers have mapped RaF’s internal structure, they can use it to probe even smaller effects that might break nature’s symmetries. The next step, Wilkins said, is to slow and trap these molecules with lasers to perform even more precise measurements.
“Now we know they can be powerful tools to look for new physics,” said Udrescu.

Larissa G. Capella is a science writer based in Washington state. She obtained a B.S. in physics and a B.A. in English creative writing in 2024, which enabled her to pursue a career that integrates both disciplines. She reports mainly on environmental, Earth and physical sciences, but is always willing to write about any science that sparks her curiosity. Her work has appeared in Eos, Science News, Space.com, among others.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus










