Electrical stimulation could treat traumatic brain injuries
An early trial suggests that deep brain stimulation could treat cognitive impairment associated with traumatic brain injury.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
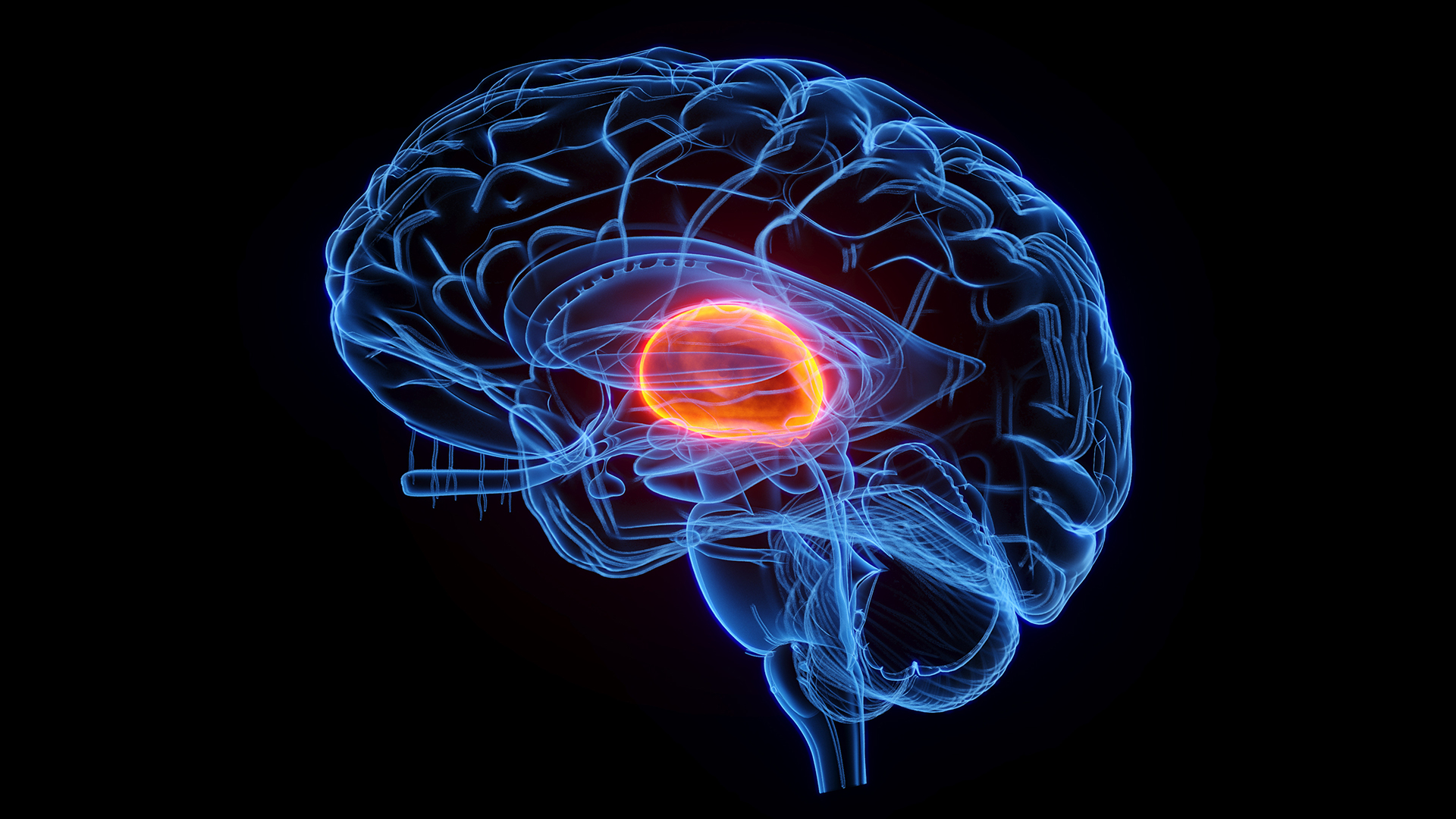
Stimulating part of the brain with electricity could improve the cognitive function of patients who've experienced debilitating traumatic brain injuries (TBI), an early clinical trial suggests.
TBI is caused by a severe blow to the head or body that damages neurons in the brain, or by an object directly penetrating the skull. The injuries range in severity, with mild cases causing temporary decline in normal brain function and more severe cases leading to longer-term impairments or even death.
In a small trial of five patients with long-lasting impairments from TBI, scientists showed that stimulating a specific brain region with surgically implanted electrodes improved the speed at which patients processed information by up to 52%.
The findings, published Monday (Dec. 4) in the journal Nature Medicine, need to be validated in larger clinical trials with more patients. However, the authors say that the results provide a "strong signal" that this approach could fill a void in the available treatments for moderate to severe TBI.
Related: Brain signals underlying chronic pain could be 'short-circuited,' study suggests
"There is no therapy that has shown efficacy for this problem [TBI] in the chronic stage," Dr. Nicholas Schiff, the lead study author and a professor of neurology and neuroscience at Weill Cornell Medicine in New York, told Live Science.
Previously, scientists had attempted to "wake up" people with TBI using drugs that influence the activity of neurotransmitters — chemicals that relay signals between neurons, Schiff said. However, none of these have been successful.
Get the world’s most fascinating discoveries delivered straight to your inbox.
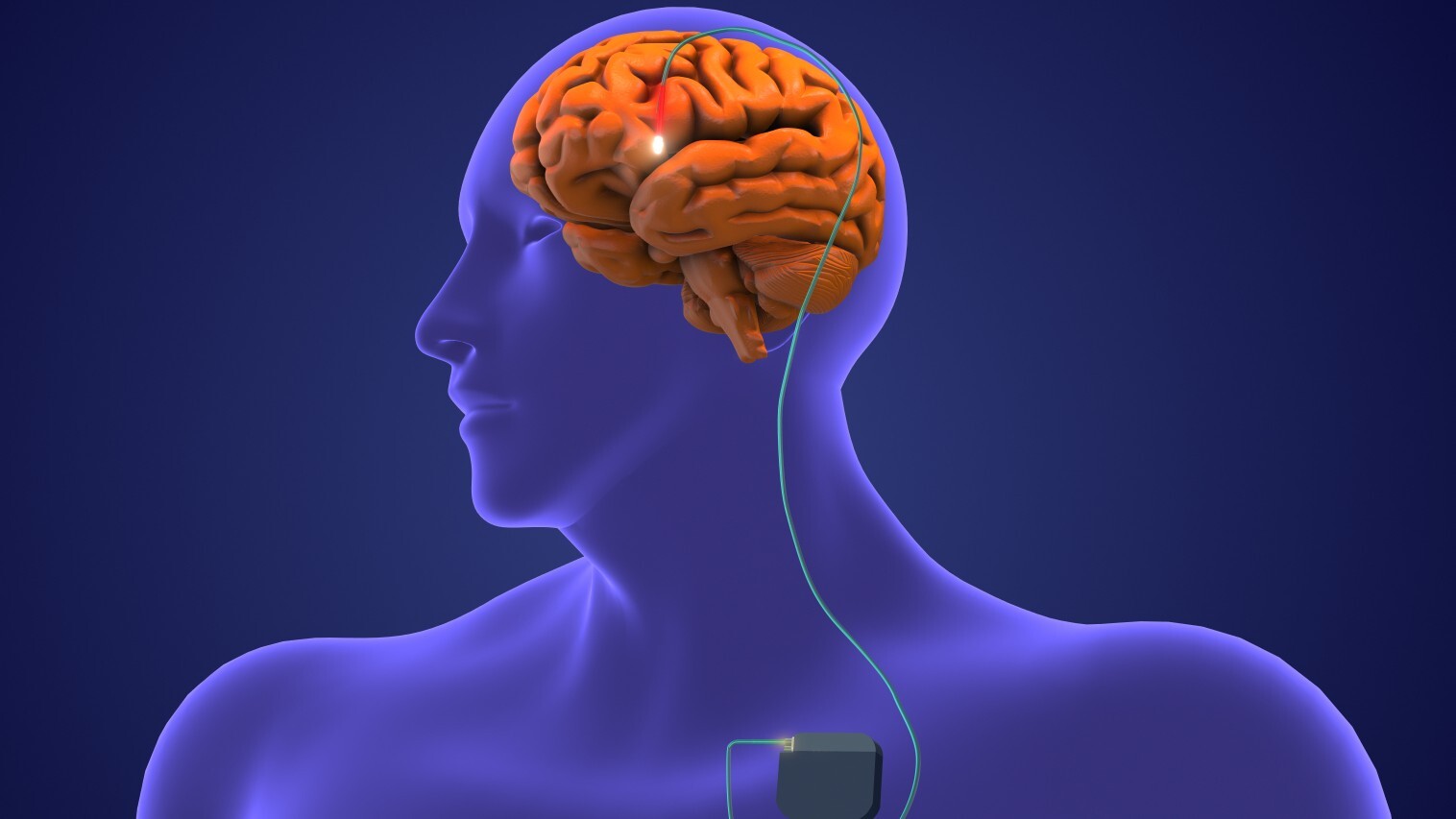
So in the new study, the researchers took a different approach. Previous research suggested that the cognitive deficits in TBI patients stems from damage to the thalamus, a key relay station for information that's important to learning and memory. Using surgically implanted electrodes, the team targeted a specific part of the thalamus called the central lateral nucleus. When impaired after TBI, the nucleus seems to contribute to a loss of executive function, meaning the ability to plan and execute tasks, and processing speed, or the ability to process information quickly.
The researchers tested the approach in six adult volunteers with chronic TBI who'd been injured around 3 to 18 years beforehand. The patients were still able to live on their own and work, but they struggled with focus and attention, organizing and planning activities and having the mental energy to complete tasks.
The researchers inserted electrodes into the central lateral nucleus of the patients' brains and stimulated this region for 12 hours a day for three months.
"By simply driving these neurons with this pacemaking at high frequencies, we can start to push them into a range of functions that they're not going to be in if they're underactivated," Schiff said.
The electrodes were safely implanted in all the patients and no serious side effects were reported. However, one patient was withdrawn from the study because they didn't comply with the protocol.
The researchers assessed the processing speed of the remaining five patients using a test where the patients had to match up a bunch of numbers and letters and put them in a specific sequence against the clock. Deep brain stimulation improved the patients' processing speeds by between 15% and 52% from baseline levels measured before surgery. This figure was above 20% for all but one person and above 40% for two people.
The trial was only designed to rule out potential safety concerns and provide early evidence that the treatment may be effective. For future trials, researchers will need to determine how to check that the stimulation leads to actual functional improvements in patients' lives, and not just improvements on cognitive tests, Schiff said. They'll also need to assess whether these initial beneficial effects may last.
But there's reason to hope that the treatment can help patients. The researchers interviewed patients and their family members before and after the trial, and what they discovered was "remarkable," Schiff said — patients were able to concentrate more effectively and in some cases be able to better engage in their school work or reflect on their job choices.
"It’s beyond my hopes, beyond anticipation," one participant's mother said. "Somebody turned the light back on."
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.
 Live Science Plus
Live Science Plus