Universe’s First Molecule Detected in Space for the First Time Ever

Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
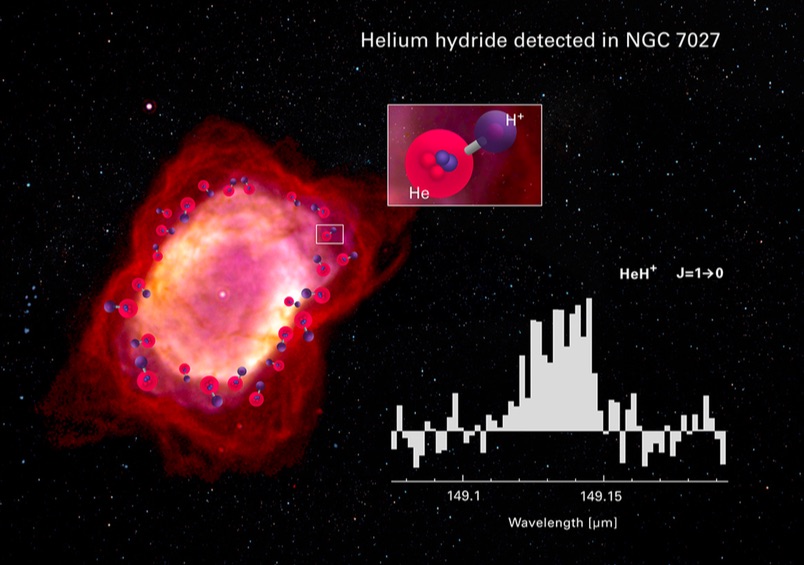
A few hundred thousand years after the Big Bang, the hot, young soup of our universe cooled enough for the smallest building blocks of life to combine into atoms for the first time. One balmy, 6,700-degree-Fahrenheit day (3,700 degrees Celsius), a helium atom glommed onto a single proton — actually a positively charged hydrogen ion — and the universe's very first molecule was formed: helium hydride, or HeH+.
Scientists have studied lab-made versions of this primordial molecule for nearly a century, but they have never found traces of it in our modern universe — until now. In a new study published today (April 17) in the journal Nature, astronomers report on their use of an airborne telescope to detect HeH+ smoldering in the cloud of gas around a dying star some 3,000 light-years away.
According to the researchers, this discovery, which has been more than 13 billion years in the making, shows conclusively that HeH+ is formed naturally in conditions similar to those found in the early universe. [5 Elusive Particles That May Lurk in the Universe]
"Although HeH+ is of limited importance on Earth today, the chemistry of the universe began with this ion," the team wrote in the new study. "The unambiguous detection reported here brings a decades-long search to a happy ending at last. "
The first molecule in the universe
HeH+ is the strongest known acid on Earth and was first synthesized in a lab in 1925. Because it is made from hydrogen and helium — the two most abundant elements in the universe and the first to emerge from the nuclear reactor of the Big Bang 13.8 billion years ago — scientists have long predicted that the molecule was the very first one to form when the cooling universe allowed protons, neutrons and electrons to exist side by side in atoms.
Scientists can't rewind the universe to hunt for this fledgling molecule where it was born, but they can look for it in parts of the modern universe that best replicate those superhot, superdense conditions — in the young nebulae of gas and plasma that explode out of dying stars.
These so-called planetary nebulae form when sun-like stars reach the end of their lives, blast away their outer shells and shrivel into white dwarfs to slowly cool into crystal balls. As those dying stars cool, they still radiate enough heat to strip nearby hydrogen atoms of their electrons, turning the atoms into the bare protons that are required for HeH+ to form.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Detecting HeH+ in even the closest planetary nebulae to Earth is tricky, because it glows at an infrared wavelength that is easily obscured by our own planet's atmosphere. In the new study, researchers got around that atmospheric haze by using a high-tech telescope mounted on a moving aircraft called SOFIA (the Stratospheric Observatory for Infrared Astronomy).
Over the course of three flights in 2016, the team trained SOFIA's telescope on a planetary nebula called NGC 7027, about 3,000 light-years from Earth. The nebula's central star is one of the hottest known in the sky, the researchers wrote, and is estimated to have shed its outer envelope only about 600 years ago. Because the surrounding nebula is so hot, young and compact, it's an ideal spot for hunting HeH+ wavelengths. According to the researchers, that's exactly where SOFIA found them.
"The discovery of HeH+ is a dramatic and beautiful demonstration of nature's tendency to form molecules," study co-author David Neufeld, a professor at Johns Hopkins University in Baltimore, said in a statement. "Despite the unpromising ingredients that are available, a mixture of hydrogen with the unreactive noble gas helium, and a harsh environment at thousands of degrees Celsius, a fragile molecule forms."
- Beyond Balloons: 8 Unusual Facts About Helium
- 15 Amazing Images of Stars
- 6 Cosmic Catastrophes That Could Wipe Out Life on Earth
Originally published on Live Science.

Brandon is the space / physics editor at Live Science. With more than 20 years of editorial experience, his writing has appeared in The Washington Post, Reader's Digest, CBS.com, the Richard Dawkins Foundation website and other outlets. He holds a bachelor's degree in creative writing from the University of Arizona, with minors in journalism and media arts. His interests include black holes, asteroids and comets, and the search for extraterrestrial life.
 Live Science Plus
Live Science Plus