Stem Cells Could Replace Hip Replacements
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
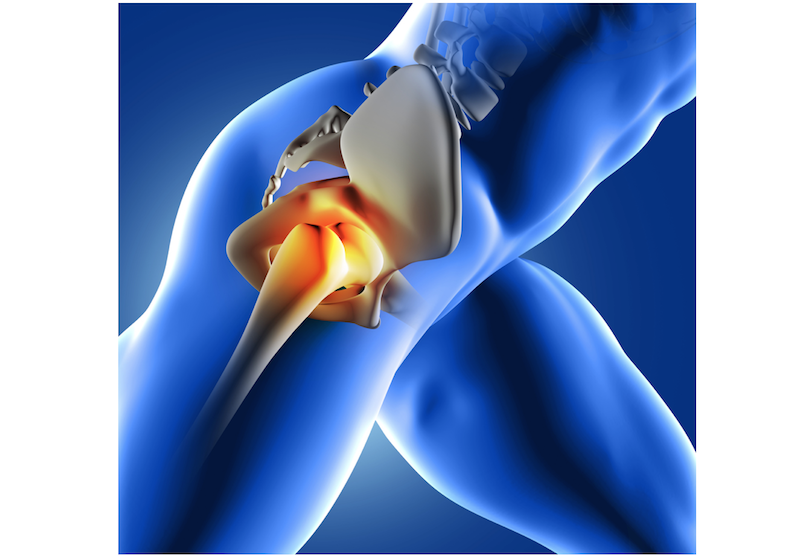
Scientists have coaxed stem cells to grow new cartilage on a scaffold shaped like the ball of a hip joint. This is a major step toward being able one day to use a patient's own cells to repair a damaged joint, thus avoiding the need for extensive joint-replacement surgery.
In addition, the scientists used gene therapy to grant this new cartilage the ability to release anti-inflammatory molecules when needed. If done in patients, this technique could help prevent a return of arthritis, if that was what damaged the joint in the first place.
The new technique may be ready to test in humans within three to five years and may ultimately work with other joints, such as knees, said Farshid Guilak, a professor of orthopedic surgery at Washington University School of Medicine in St. Louis, who co-led the project. [5 Amazing Technologies That Are Revolutionizing Biotech]
The work, a collaboration between researchers at Washington University in St. Louis and researchers from Cytex Therapeutics, Inc. in Durham, North Carolina, appears today (June 18) in the journal Proceedings of the National Academy of Sciences.
The researchers said that the stem cell therapy might be particularly useful to younger people who have advanced osteoarthritis. In this degenerative joint disease, the cartilage that cushions the joint between two bones wears thin due to injury, excessive use or genetic susceptibility.
More than 27 million Americans have osteoarthritis, according to the Centers for Disease Control and Prevention (CDC). The prevalence of the condition is rising and is significantly higher among obese adults; weight loss of as little as 11 lbs. (5 kilograms) reduces the risk of developing knee osteoarthritis by half, according to CDC data. [Special Report: The Science of Weight Loss]
Doctors often recommend joint-replacement surgery for severe osteoarthritis when preventive measures such as weight management, exercise and medication fail. But doctors are reluctant to perform joint-replacement surgery on patients under the age of 50, because prosthetic joints typically last for less than 20 years, and a follow-up joint-replacement surgery is risky, Guilak said.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Some doctors in private practice began to treat osteoarthritis with stem cells as early as 2008. Mostly, doctors did this by simply injecting stem cells directly into the affected area with the hope that the cells would latch onto the joint, turn into cartilage and provide cushioning for the joint. But this method has never been shown to have a benefit, and the Food and Drug Administration (FDA) has questioned the safety and efficacy of the untested therapy, which can cost thousands of dollars per injection. The method has, however, been popular among athletes.
In 2014, a U.S. Court of Appeals decision upheld the FDA's authority to regulate stem cell treatment. That decision prevented U.S.-based clinics from offering these stem cell injections. Meanwhile, researchers have sought more-legitimate ways in which stem cells could be used for regenerative medicine. [11 Body Parts Grown in the Lab]
"Our study has made several major advances," Guilak told Live Science. "One important breakthrough has been to create cartilage that has the same load-bearing properties as normal cartilage but … [is] grown outside the body using stem cells from fat."
Most previous studies targeted small defects in the cartilage, an approach that was like trying to fix "a pothole in the road," he said. But in the new study, the researchers created a replacement that is the size of the entire joint. "We have the potential to resurface the whole hip in the case of osteoarthritis, which is much more common than a small defect," he said.
Guilak said the procedure is straightforward: Stem cells are extracted from a patient's fat and seeded on an external woven scaffold, designed to fit over the ball of the patient's joint. Using a "cocktail of proteins," Guilak said, stem cells are coaxed into turning into cartilage cells, before spreading throughout the woven scaffold over a period of six weeks.
The plan is to remove the worn-out cartilage from the ball of the joint and replace this with a "living joint" to resurface the hip, Guilak said. This plan hasn't been performed on humans yet but will be tested now in animals.
"Unlike a standard metal and plastic prosthesis, the bone of the hip is kept intact, and the surgery is much less invasive," Guilak said.
The scaffold in which the cartilage cells grow is a unique structure consisting of approximately 600 biodegradable fiber bundles woven together to create a high-performance fabric that can function like normal cartilage.
"The woven implants are strong enough to withstand loads up to 10 times a patient's body weight, which is typically what our joints must bear when we exercise," said Franklin Moutos, vice president of technology development at Cytex and the first author on the journal article.
Guilak said that the ultimate success of this new technique may depend on the gene-therapy element, which would help thwart new damage to the new cartilage. "We have modified the stem cells genetically to give them the ability to release anti-inflammatory drugs on demand, which has not been done in bioartificial cartilage previously," he said.
In this way, the joint would have a built-in medicine cabinet and perhaps be better than new, Guilak said.
Follow Christopher Wanjek @wanjek for daily tweets on health and science with a humorous edge. Wanjek is the author of "Food at Work" and "Bad Medicine." His column, Bad Medicine, appears regularly on Live Science.

Christopher Wanjek is a Live Science contributor and a health and science writer. He is the author of three science books: Spacefarers (2020), Food at Work (2005) and Bad Medicine (2003). His "Food at Work" book and project, concerning workers' health, safety and productivity, was commissioned by the U.N.'s International Labor Organization. For Live Science, Christopher covers public health, nutrition and biology, and he has written extensively for The Washington Post and Sky & Telescope among others, as well as for the NASA Goddard Space Flight Center, where he was a senior writer. Christopher holds a Master of Health degree from Harvard School of Public Health and a degree in journalism from Temple University.
 Live Science Plus
Live Science Plus










