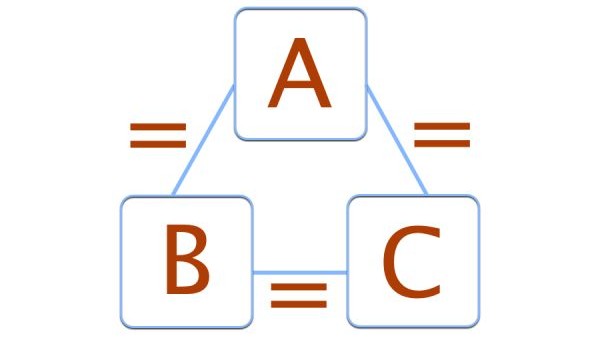
What is the zeroth law of thermodynamics?
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with a third body, they are also in equilibrium with each other.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
The zeroth law of thermodynamics states that if two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. Thermal equilibrium means that when two bodies are brought into contact with each other and separated by a barrier that is permeable to heat, there will be no transfer of heat from one to the other.
In other words, the zeroth law means the three bodies are all the same temperature, according to NASA. James Clerk Maxwell put this perhaps more simply when he said, "All heat is of the same kind." (Longmans, Green, and Co. 1875). What is most important is that the zeroth law establishes that temperature is a fundamental and measurable property of matter.
Related: 5 sci-fi concepts that are possible (in theory)
History of the zeroth law of thermodynamics
When the zeroth law was originally conceived in the 18th century, there were already two laws of thermodynamics. .However, this new law, which presented a formal definition of temperature, actually superseded the existing laws and should rightfully be at the head of the list, according to OpenStax, an educational organization run by Rice University. This created a dilemma: The original laws were already well known by their assigned numbers, and renumbering them would create a conflict with the existing literature and cause considerable confusion. One scientist, Ralph H. Fowler, came up with a solution to the dilemma: He called the new law the "zeroth law." (Cambridge University Press, 1939). (Interestingly, science-fiction writer Isaac Asimov appropriated the idea for a zeroth law in his 1985 novel "Robots and Empire" when he found that he needed to add a new law to the Three Laws of Robotics that superseded the First Law.)
David McKee, a professor of physics at Missouri Southern State University, told Live Science that the zeroth law establishes that "the temperature of two systems is the only thing you need to know in order to determine which direction heat will flow between them."
Thermometers
The zeroth law of thermodynamics defines temperature and makes thermometers possible. For a thermometer to be useful, though, it must be first calibrated. All other basic units of measure, — such as for length, mass, time, etc. — are each defined according to a precise standard. In this case, scientists must not only define a unit of measure, but also the beginning point of the scale.
The most notable early efforts to standardize temperature measurement were those of German instrument maker Daniel Gabriel Fahrenheit. In the early 18th century, Fahrenheit invented the familiar glass-tube-type thermometers using both alcohol and mercury. He also invented the Fahrenheit scale, which sets the freezing and boiling points of water at 32 degrees Fahrenheit and 212 degrees Fahrenheit, respectively, and is still used to this day, particularly in the United States. Most of the rest of the world uses the Celsius scale, which assigns values of 0 degrees for the freezing point of water and 100 degrees for its boiling point at mean sea level.
Get the world’s most fascinating discoveries delivered straight to your inbox.
All measuring scales used in science and engineering start at a value of zero. The concept of zero length, mass or time is relatively easy to grasp; however, zero temperature, or absolute zero, where there is absolutely no heat energy whatsoever, is a bit more difficult to grasp. This is because such a temperature has never been observed in nature or in the laboratory, and it is generally believed that it never will be; however, scientists have gotten very close.

The unit for thermodynamic temperature is the kelvin (K) and was originally defined according to the triple point of water. The triple point is defined as "
the condition of temperature and pressure under which the gaseous, liquid, and solid phases of a substance can exist in equilibrium," according to Merriam-Webster. While this standard was convenient for ambient temperatures, it made measurements at very high and very low temperatures uncertain. So in 2019, The International Bureau of Weights and Measures changed the standard to be based on the Boltzmann constant (k), which relates temperature to the average energy of the particles in a substance. That definition acknowledges that temperature is a measure of the average energy of molecular motion, according to the U.K.'s National Physical Laboratory.
Most thermometers contain liquid or metal that changes volume or shape depending on its temperature. When the liquid or metal reaches thermal equilibrium with the object or substance being measured, the temperature-sensitive property of the material in the thermometer can then be exploited to indicate its temperature, according to NASA.
For instance, some types of thermometers use a liquid, typically alcohol or mercury, which expands or contracts with increasing or decreasing temperature. This small expansion is amplified by having a relatively large reservoir of liquid in a glass bulb connected to a long and very narrow glass tube. In this way, a small change in the volume of the liquid in the bulb can cause a large change in the level of the liquid in the tube; the temperature can then be determined by reading the height of the liquid against a calibrated scale, according to Harper College.
Another type of thermometer relies on the thermal expansion of metal. Again, the problem is how to amplify a very small change in size so it can be read on a scale. One method involves a coil with many loops, so that even a slight change in length gets multiplied by the number of loops and so is readable. Another method exploits the fact that different metals expand at different rates when heated. Strips of two different metals with different coefficients of expansion — the degree to which the material expands for a certain amount of temperature change — can be laminated together so that the assembly will curl when heated. This "curling" can move a needle that can be read against a scale, according to The Pennsylvania State University.
Another method for measuring temperature involves color changes in temperature-sensitive organic materials. These are typically useful only for measuring limited temperature ranges, such as indicating fever or monitoring room temperature. Another device, called a thermistor, works based on changes in the electrical resistivity of a semiconducting material due to its temperature, according to Penn State. These devices can detect extremely small temperature changes and are used in bolometers — which measure radiation based on a change in temperature — and to monitor laboratory experiments. However, no measurement would be possible without relying on the principle described in the zeroth law.
This article was updated on Feb. 1, 2022 by Live Science contributor Ashley Hamer.
Additional resources
- This video from CrashCourse is a colorful, animated explainer about the first and zeroth laws of thermodynamics.
- Learn about the laws of thermodynamics from the "Great Explainer" in this Richard Feynman lecture, courtesy of CalTech.
- John Horgan says that the so-called "minus-first law" is even more important than the zeroth law of thermodynamics in this opinion piece in Scientific American.
Bibliography
NASA Glenn Research Center, "Thermodynamic Equilibrium (Zeroth Law)," 2021. https://www.grc.nasa.gov/WWW/K-12/airplane/thermo0.html
James Clerk Maxwell, "Theory of Heat," Longmans, Green, and Co. 1875.
Ian T. Durham, "Ralph Fowler," St. Anselm College, March 2001. https://mathshistory.st-andrews.ac.uk/Biographies/Fowler/
Biography, "Isaac Asimov," April 2014. https://www.biography.com/writer/isaac-asimov
MindMatters News, "The Three Laws of Robotics Have Failed the Robots," September 28, 2019. https://mindmatters.ai/2019/09/the-three-laws-of-robotics-have-failed-the-robots/
University of Michigan, "The Three Laws of Robotics." http://websites.umich.edu/~engb415/literature/cyberzach/Asimov/robolaw.html
Encyclopedia of World Biography, "Gabriel Fahrenheit Biography." https://www.notablebiographies.com/Du-Fi/Fahrenheit-Gabriel.html
Merriam-Webster Dictionary, "Triple Point." https://www.merriam-webster.com/dictionary/triple%20point
The International Bureau of Weights and Measures, "SI Brochure: The International System of Units (SI)," 2019. https://www.bipm.org/en/publications/si-brochure
National Physical Laboratory, "SI Units: kelvin (K)," 2021. https://www.npl.co.uk/si-units/kelvin
Harper College, "Measuring Temperature." http://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/labtech/temper.htm
The Pennsylvania State University, "Meteorology 3: Thermometers." http://www.meteo.psu.edu/wjs1/Meteo3/Html/temperature.htm
The Pennsylvania State University, "NTC Thermistors." https://www.me.psu.edu/sommer/me445/n11_thermistors.pdf
University of California, Berkeley, "Why bolometers?" 2001. http://bolo.berkeley.edu/bolometers/introduction.html

- Ashley HamerLive Science Contributor
 Live Science Plus
Live Science Plus





