Getting a Head: How Worms Regenerate Lost Tissue

The mystery of why some animals can regenerate body parts while others cannot has long puzzled scientists, but three new studies have brought the issue to a head.
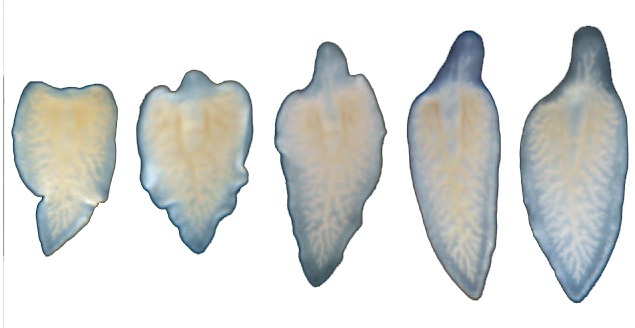
Three different research groups studied why some species of flatworms can regenerate entire heads and tails after being cut into pieces, while other species of worm only partially regenerate their body parts. By activating a single gene in the cascade of signals involved in regeneration, the researchers restored the worms' ability to grow new heads.
"In flatworms, you can cut out a single piece from any part of the worm, and somehow, magically, it turns itself into a worm," said molecular biologist Jochen Rink, who led one of the studies. The question is, "Why can some animals regenerate while others can't?" said Rink, of the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, Germany.
Undead head
To find out the answer to this question, the three groups studied several closely related species of flatworm, or planarian. Some species, such as Schmidtea mediterranea, have superb regeneration abilities. If you cut these worms anywhere along their length, the head portion will grow a new tail, and the tail portion will grow a new head. [Image Gallery: Remarkable Bionic Animals]
In contrast, species such as Dendrocoelum lacteum have less capacity for regeneration. If you cut these worms within the quarter of their body closest to their head, they will form two complete worms, but if you cut them farther down, the intact tail portion will not regrow a head.
The process of growing a new head, complete with a brain and eyes, is complex, to say the least. Previous research has shown that severing a body part sets off a chain of signals in stem cells, the biological putty that can develop into any tissue type. Together, these signals act as a molecular switch: Activating the signals leads to the creation of a new tail, whereas blocking them enables a new head to develop.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Now, using cutting-edge genetic-sequencing technologies, the researchers determined which genes were active, turning on regeneration signals, in the flatworms that could generate new noggins compared with those that couldn't.
None of the genes associated with head regeneration was active in the tail halves of those worms that only partially regenerated, the studies found. "It was as if the [tail] piece never made the decision to initiate head regeneration," Rink told LiveScience.
Next, the researchers artificially blocked the molecular signals that cause tail regeneration. As a result, the worms that previously lacked the ability to regenerate heads were able to grow new ones.
The idea that interfering with a single gene could overcome the blockage of head regeneration was a big surprise, said Phillip Newmark, a developmental biologist at the University of Illinois at Urbana-Champaign and leader of one of the other studies.
Rink agreed. "In order to induce this process artificially, you might think you would have to twiddle a hundred knobs at the same time — but you may have to only twiddle a few knobs," he said.
Flatworms can even be made to grow a second head in place of a tail, and each head controls half of the body. Or, you can make a worm with no head and two tails, which makes you wonder why they need a brain at all, Rink said.
Powers of regeneration
The teams can only speculate as to why some flatworm species can regenerate heads naturally but other worm species cannot. There could be trade-offs to complete regeneration ability, the researchers said. Rink speculated that some flatworms use regeneration as a method of asexual reproduction. "The animal attaches to the surface, and the head 'walks off'," he said. Other flatworms may concentrate on producing eggs for sexual reproduction, at the expense of their ability to regenerate.
Neurobiologist Alejandro Sánchez Alvarado, who was not involved in any of the studies, said the findings are important to scientists' understanding of regeneration in flatworms and the phenomenon's evolution. "The question remains whether this type of modulation may or may not explain loss or gains of regenerative capacities in animals other than [flatworms]," said Sánchez Alvarado, a neurobiologist at the Stowers Institute for Medical Research in Kansas City, Mo.
Other animals, such as certain types of salamanders and fish, can also regenerate limbs. But it's not yet clear whether they use the same "blueprint" for regeneration or whether they evolved their abilities independently, Rink said.
It's tempting to ask whether humans might benefit from these studies of tissue regeneration. But humans are very different from flatworms and don't have the ability to partially regenerate as the worms do.
"Exploring how we can translate principles derived from these studies to those of mammalian regeneration would be interesting and may eventually be beneficial for development of regenerative medicine [in humans]," said Mayumi Ito, a professor of dermatology at NYU School of Medicine who was not involved with the studies.
All three groups' findings were detailed online today (July 24) in the journal Nature.
Follow Tanya Lewis on Twitter and Google+. Follow us @livescience, Facebook & Google+. Original article on LiveScience.com.

 Live Science Plus
Live Science Plus