Storm Clouds May Punch Holes in Ozone

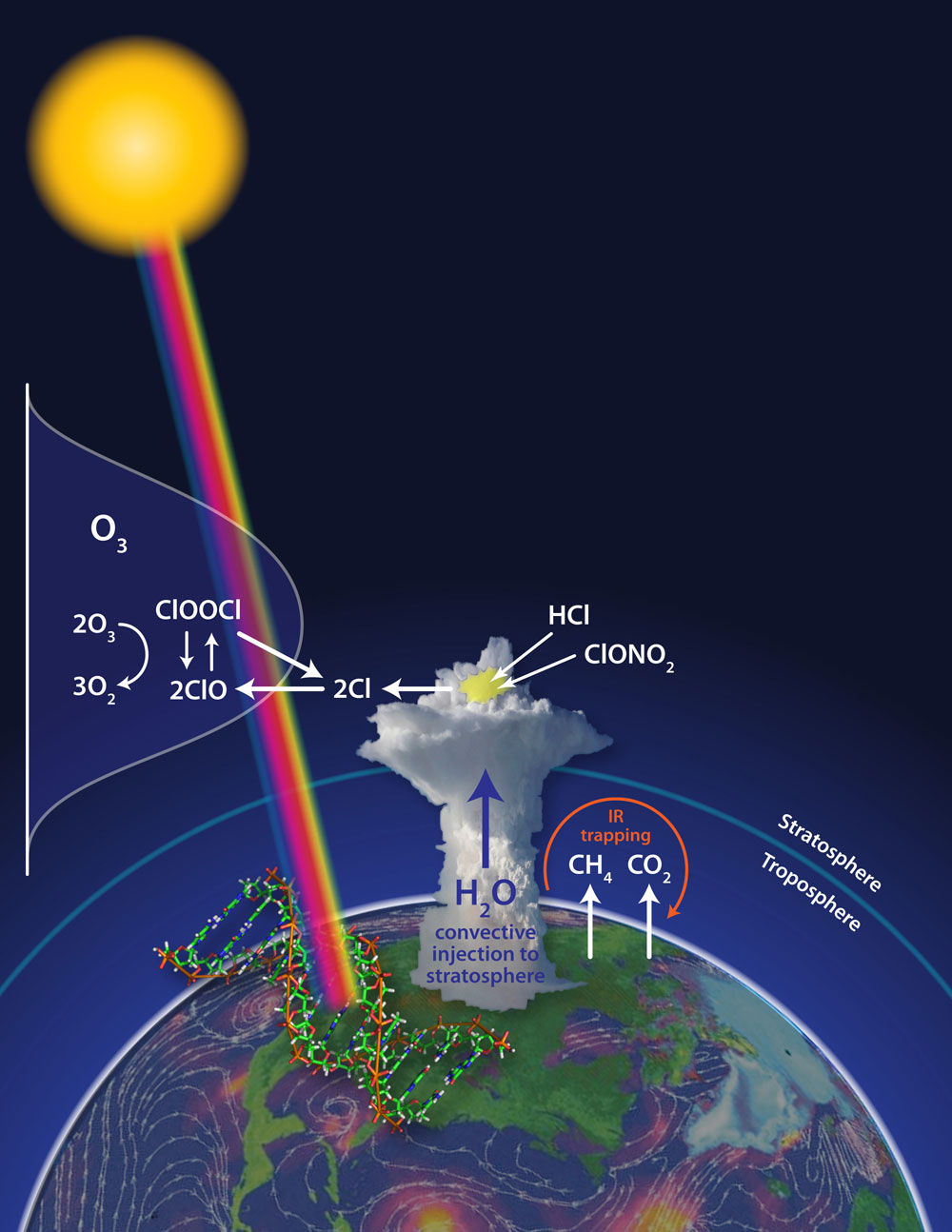
The same process that creates an ozone hole over Antarctica shows up above big summer storm clouds in the United States and could be destroying ozone there, a new study proposes.
Ninety percent of Earth's ozone is in the stratosphere (the second layer of the atmosphere, just above the one we breathe, the troposphere). This ozone forms the ozone layer, which protects everything on the Earth's surface from the sun's harmful ultraviolet rays.
Ozone destruction in the lower stratosphere is caused by reactive chlorine and bromine molecules called free radicals. The free radicals steal one of ozone's three oxygen atoms. Losing an atom transforms ozone into an everyday oxygen molecule that doesn't confer the same protective benefits.
Usually, the chemical reactions that zap ozone happen only at extremely cold temperatures — about minus 112 degrees Fahrenheit (minus 80 degrees Celsius) — such as high in the atmosphere above the frozen poles. But Jim Anderson, lead author of the new study, suspected increasing the concentration of water vapor in the stratosphere through storm clouds meant the chemical reactions would spark at higher temperatures seen above the mid-latitudes of the United States.
"Anytime you satisfy the proper concentration of water and temperature, these reactions will take place," said Anderson, an atmospheric chemistry professor at Harvard University.
Water injections
Towering cumulonimbus clouds, the culprit behind severe storms, carry water vapor high into the sky via convection. During monitoring flights conducted between 2001 and 2007, scientists found the cloud tops reached into the lower stratosphere, at altitudes between 9 and 12 miles (15 and 20 kilometers) above us, sending the water they contained aloft. [Infographic: Layers of Earth's Atmosphere]
Get the world’s most fascinating discoveries delivered straight to your inbox.
"These convective storms inject water far more deeply into the stratosphere than anybody ever suspected, and that immediately began to concern us," Anderson told OurAmazingPlanet.
Anderson's key concern is chlorine free radicals. The atoms assume their dangerous nature during a rapid series of chemical reactions that take place on the surface of sulfate-water aerosols, tiny droplets that are ubiquitous in the lower stratosphere.
"The chemistry of these sulfate-water aerosols is such that conversion rate from inorganic chlorine is a function not just of temperature, but of water vapor and temperature," Anderson said. Adding more water vapor to the lower stratosphere means chlorine free radicals can form at higher temperatures, he explained. The abundant sunlight available at lower latitudes also boosts the reaction speed.
The study shows chlorine molecules build up rapidly, within the first 24 hours after a storm. As a result, ozone loss can increase by two orders of magnitude compared to that in the regularly arid stratosphere.
"We were quite surprised by the rate that the chemistry responds to the sunlight conditions in the lower stratosphere," Anderson said. "This conversion takes place in one diurnal period, and that's a lot more rapidly than we had expected."
Direct observations needed
Computer modeling, combined with data from research aircraft, indicates the right conditions occur above the central United States during summertime, the new study finds.
However, no one has directly observed the process taking place. That makes other scientists intrigued but cautious about the theory.
"I don't think Jim's proven the case yet," said Andrew Dessler, professor of atmospheric chemistry at Texas A&M University. "We have no data to refute or support the idea."
Mary Barth, a scientist with the National Center for Atmospheric Research, said Anderson's paper will encourage the research community to "look at things a little differently."
"Understanding how much stuff gets into the stratosphere is something we're still figuring out in detail. What he is doing is pushing us to really evaluate his theory and get some data to see if it makes sense," she said.
Anderson and his co-authors call on NASA to fly research aircraft through stratospheric cloud tops to confirm evidence of ozone hole chemistry in their paper, published today (July 26) in Science Express, the online edition of the journal Science
"We have a very powerful foundation of chemistry that we have verified through many observations of a wide range of conditions," Anderson said. "It's true that if we had our way, we would like to observe this within a convective event over the United States."Future research could also show whether the process is confined to storms, or if water vapor circulates in the stratosphere, allowing free radicals to deplete ozone over a wide area.
"If the air blows downwind and it's gone, then this is a curiosity," Dessler said. "On the other hand, there might be enough water vapor coming out of these events to have a detectable effect on ozone. If it's happening, we want to know if this is important on a hemispheric scale or does this have an effect on global ozone."
This story was provided by OurAmazingPlanet, a sister site to LiveScience.