'Miniantibodies' Reduce Inflammation and Pain
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
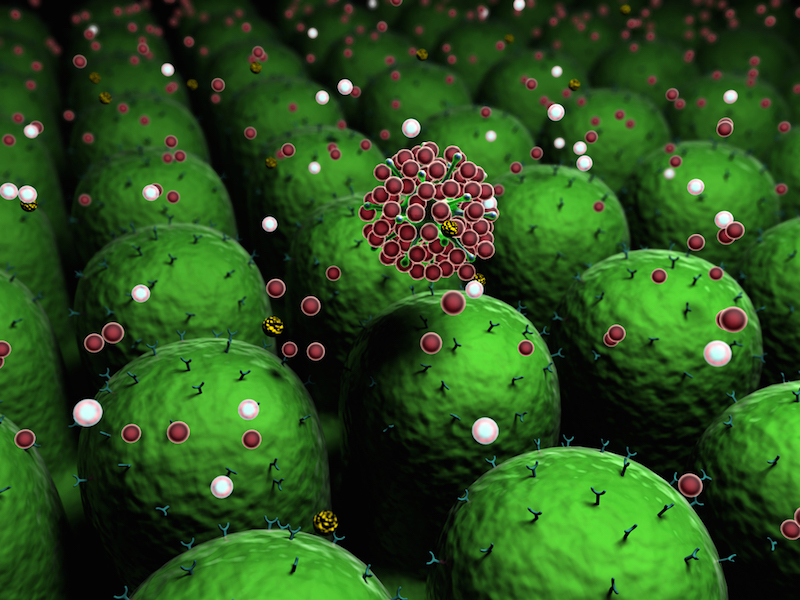
Researchers in Europe say a new type of biological molecule called nanobodies, or miniantibodies, can block inflammation and reduce pain in mice — a technique they describe as a next-generation strategy against inflammatory diseases.
In experiments on mice, the nanobodies appeared to be more effective at controlling inflammation than either regular antibodies or the anti-inflammatory drugs that are typically used, the researchers said. The nanobodies could one day be a potent treatment for chronic pain, inflammatory bowel disease, multiple sclerosis and other inflammatory disorders, they said.
The research team also tested these nanobodies in a sample of human blood. The team members found that the nanobodies were 1,000 times more potent in hindering the release of inflammatory molecules than similar, small-molecule drug candidates. [The 7 Biggest Mysteries of the Human Body]
The research, led by Friedrich Koch-Nolte of the University Medical Center Hamburg-Eppendorf in Germany, is detailed in a paper that was published today (Nov. 23) in the journal Science Translational Medicine.
Inflammation is an essential part of the way the human immune system works. It's an innate response to injury in which the body sends white blood cells and other molecules to repair damaged tissue or to ward off any invading pathogens.
Prolonged or chronic inflammation, however, can lead to numerous diseases and conditions, such as allergies, asthma and atherosclerosis. It can also lead to many autoimmune diseases such as rheumatoid arthritis and type 1 diabetes, in which the immune system — for reasons that are not understood — attacks the body's own tissue as if that tissue were something foreign.
Scientists are attempting to ease the symptoms of these diseases by controlling inflammation. One sought-after target has been a structure in cells called P2X7, which acts like a gateway or channel for molecules to pass in and out of cells and triggers the inflammation response.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Scientists have tried to develop drugs that block P2X7, but they have had little success. The problem is that the drugs that have been developed to date don't bind only with P2X7. And because they interact with other molecules in cells, the drugs tend to produce unwanted side effects. Meanwhile, antibodies — which are proteins that can be very effective at precisely bonding with most molecules that are found on cell surfaces — tend not to effectively block channel molecules like P2X7. [11 Surprising Facts About the Immune System]
Consequently, in the editorial summary that accompanied the new journal article, P2XZ was described as a "tough target."
Enter the nanobody. A nanobody is a fragment of an antibody — about a tenth of its size — that was developed 15 years ago from special antibodies found only in camels, llamas and alpacas. Nanobodies are like antibodies in that they can be very selective in what they bind to, thus reducing side effects.
"Nanobodies have special properties that stand out from conventional antibodies," Koch-Nolte told LiveScience. "They are fully biodegraded into non-toxic products."
Koch-Nolte's group has designed nanobodies that specifically inhibit P2X7 on immune cells — the first nanobodies to block such a channel protein.
In experiments that were performed on mice with kidney inflammation and an itchy skin condition called allergic contact dermatitis, injections of one type of nanobody reduced inflammation and relieved such disease symptoms as pain with no apparent side effects. Koch-Nolte's group then tested a similar nanobody in a sample of human blood with immune cells. The nanobody blocked P2X7 at a rate that was 1,000 times more effective than current drugs that target P2X7.
Because P2X7 is implicated in a host of inflammatory diseases, blocking its effect can have tremendous therapeutic potential for the millions of people who suffer from such diseases, the researchers said.
Follow Christopher Wanjek @wanjek for daily tweets on health and science with a humorous edge. Wanjek is the author of "Food at Work" and "Bad Medicine." His column, Bad Medicine, appears regularly on Live Science.

Christopher Wanjek is a Live Science contributor and a health and science writer. He is the author of three science books: Spacefarers (2020), Food at Work (2005) and Bad Medicine (2003). His "Food at Work" book and project, concerning workers' health, safety and productivity, was commissioned by the U.N.'s International Labor Organization. For Live Science, Christopher covers public health, nutrition and biology, and he has written extensively for The Washington Post and Sky & Telescope among others, as well as for the NASA Goddard Space Flight Center, where he was a senior writer. Christopher holds a Master of Health degree from Harvard School of Public Health and a degree in journalism from Temple University.
 Live Science Plus
Live Science Plus











