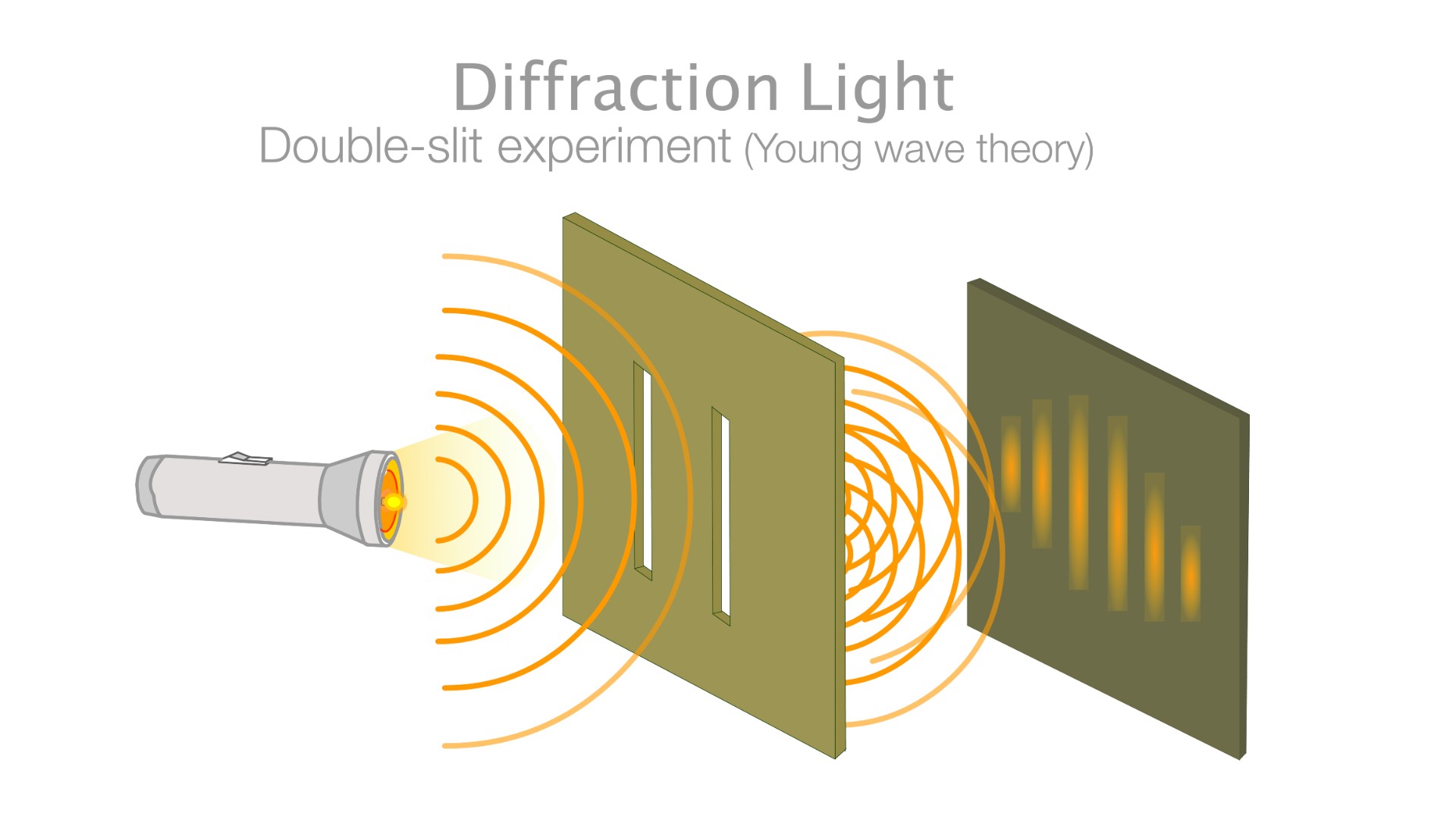
Is all matter made up of both particles and waves?
According to quantum mechanics, the physics theory that describes the zoo of subatomic particles, all matter can be described as both particles and waves. But is it real?
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Wave-particle duality is a cornerstone of quantum mechanics, which is our modern theory of how subatomic particles behave. The duality states that all particles (in fact, all objects) have a wave-like property associated with them. While most standard interpretations of quantum mechanics treat this wave-like property as a neat mathematical trick for calculating the probability of a particle being in one place or another, some more radical interpretations elevate the wave to a real entity that exists just as much as particles do. One of these interpretations, known as the pilot-wave theory, posits that every interaction in the universe can be described by a single set of equations — if only we could figure those out.
The original waves
In 1924, French physics doctoral student Louis de Broglie proposed that matter has a wave-like property. De Broglie was inspired by the discovery of wave-particle duality in light. For centuries, physicists had debated whether light was made of tiny particles or some sort of waves. In the mid-1800s the debate seemed to be settled with James Clerk Maxwell’s discovery of the electromagnetic field, and his realization that light was made of electromagnetic waves, according to "The History of Quantum Mechanics" (CreateSpace Independent Publishing, 2017).
But in 1899, German physicist Max Planck was trying to understand a kind of radiation known as blackbody radiation (the spectrum of light emitted by a heated object), and the only way he could explain its properties was if he allowed for light to be emitted in discrete little chunks, or quanta — meaning the emitted light could be described both as particles and electromagnetic waves.
A few years later, Albert Einstein proposed that it isn’t just the emission of light that is quantized, but light itself. According to Einstein, light is made of little particles (eventually named photons), and when a bunch of photons get together they act like a wave. This wave-particle duality, although radical, could explain a host of experiments and observations.
Related: What God, quantum mechanics and consciousness have in common
Planck and Einstein proposed that the wavelength of light is inversely proportional to its momentum. So the more momentum (energy) a photon has, the smaller its wavelength. De Broglie took this simple relationship and got a seemingly crazy idea. Light has momentum and energy, and it has a wave-like property. Matter also has momentum and energy, so perhaps it also has a wave-like property.
Nervous, de Broglie’s thesis advisor sent the idea over to Einstein, who gave it his stamp of approval.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Quantum waves
While the development of quantum mechanics had begun with Planck’s work, progress stalled for a couple decades. De Broglie’s idea was the catalyst needed to bring quantum theory into its modern form. With the realization of wave-particle duality, physicists like Erwin Schrödinger could develop a full quantum theory that explained the behavior of electrons inside of atoms, according to Erwin Schröodinger's landmark 1926 paper.
But the question remained: What exactly are waves of matter? Schrödinger argued that subatomic particles like electrons are literally smeared across space, but his interpretation didn’t agree with experiments that showed electrons to be point-like particles. Then German physicist Max Born proposed an idea that would eventually evolve into the so-called Copenhagen interpretation of quantum mechanics: Matter waves are waves of probability that indicate where someone can find particles the next time they look for them. Where the waves have high values, there is a strong likelihood of finding a particle.
Pilot waves
De Broglie had a different idea. He also believed that the waves are real, but not as a replacement for the particles. Instead, he believed they exist alongside the particles, propagating through space and telling the particles where to go. No one could see the true path of the particle trajectories, which meant particles seemed to be guided by quantum randomness, but de Broglie argued that there was no randomness involved. Instead: “pilot waves” always knew what to do.
De Broglie would eventually drop this idea in favor of the waves-as-probabilities interpretation, but decades later American physicist David Bohm picked it up again and fleshed it out into a full interpretation of quantum mechanics. Other physicists became attracted to the pilot-wave theory because it solved some of the major philosophical headaches plaguing the Copenhagen interpretation. In that interpretation, the trajectory of a particle exists in some vague quantum superposition that exists in our mathematics — we don’t really “know” what quantum particles actually do, and we can only guess at the probabilities of certain results happening.
In contrast, pilot-wave theory has the advantage that everything in the theory is real and determined — no probabilities here. Instead, in pilot-wave theory (also known as Bohmian mechanics), the wave associated with each particle moves through space, doing what waves naturally do (reflecting, interfering, spreading out, and so on). What seems to be quantum randomness to an observer is just a reflection of the fact that the particle’s true trajectory is hidden.
However, this reality must come at a price. When two particles interact, their pilot waves interact and entangle the particles. Entanglement is a quantum mechanical process in which the fates of subatomic particles are linked, even across vast distances, so that a change in one will instantly result in a change to the other. This requires a single wave equation to describe both particles simultaneously, and they must mysteriously “know” what the other one is up to, regardless of how far apart they are. This is a standard result in quantum mechanics and not too surprising. But in pilot-wave theory, this entanglement extends to the positions of the particles themselves.
As all particles are entangled with all other particles in the universe, there is a single wave equation that describes all particles simultaneously, and somehow ties their positions together. This means that in pilot-wave theory, the motions of the atoms in your body are affected by the behavior of every single particle in the entire universe. — and there exists a single equation that can tie everything in the universe together.
While this implication doesn’t rule out pilot-wave theory, it does make the proposal difficult to accept as a proper theory of physics – after all, how could we ever claim with confidence that we have isolated all the behaviors inside an experiment and understand what’s going on?
Emphasizing the positions of particles, and not their velocities, also makes it difficult to make the theory fully compatible with relativity, which says that positions and velocities must be treated on an equal footing. Without this compatibility, it’s difficult to expand pilot-wave theory into all the varieties of physical systems that quantum theory applies to (like high-energy particle colliders).
There could be an answer to these difficulties one day, and pilot wave theory has experienced growing interest over the past few decades. But until the question is fully resolved, the reality of wave-particle duality remains a matter of taste.
This is part of an ongoing series describing potential interpretations of quantum mechanics.

Paul M. Sutter is a research professor in astrophysics at SUNY Stony Brook University and the Flatiron Institute in New York City. He regularly appears on TV and podcasts, including "Ask a Spaceman." He is the author of two books, "Your Place in the Universe" and "How to Die in Space," and is a regular contributor to Space.com, Live Science, and more. Paul received his PhD in Physics from the University of Illinois at Urbana-Champaign in 2011, and spent three years at the Paris Institute of Astrophysics, followed by a research fellowship in Trieste, Italy.
 Live Science Plus
Live Science Plus