Scientific breakthrough leads to 'fluorescent biological qubit' — it could mean turning your cells into quantum sensors
Fluorescent proteins can be turned into qubits within cells and could give us a deeper understanding of biology at the nanoscale level.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Protein-based quantum bits (qubits) could be the key to accelerating biological research at the smallest of scales, thanks to a new scientific breakthrough.
Researchers from the University of Chicago have discovered a way to turn a fluorescent protein into a biological qubit that can be built directly inside a cell, then used as a way to detect magnetic and electrical signals within the cell. This breakthrough was detailed in a paper published Aug. 20 in the journal Nature.
"Our findings not only enable new ways for quantum sensing inside living systems but also introduce a radically different approach to designing quantum materials,” said Peter Maurer, co-principal investigator and assistant professor of molecular engineering at UChicago, in a statement. “Specifically, we can now start using nature’s own tools of evolution and self-assembly to overcome some of the roadblocks faced by current spin-based quantum technology.”
By developing biological qubits that can be deployed inside cells using existing proteins already employed in microscopy, this research bypasses the need to retrofit existing quantum devices to work in biological systems. This could eventually lead to quantum sensors that don’t need the extreme cooling and isolation normally needed for quantum technology.
Fluorescent findings
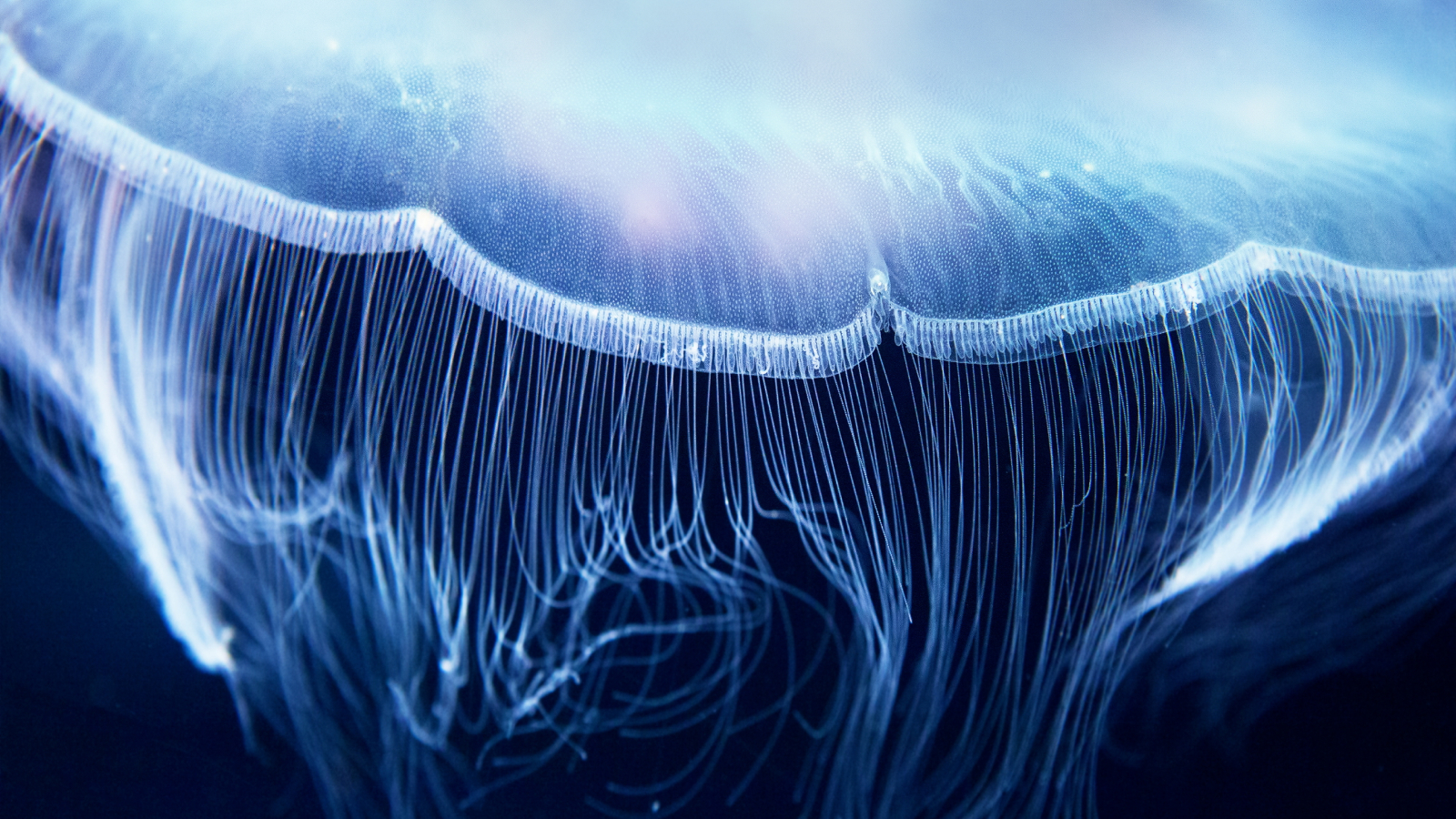
Fluorescent proteins, which can be found in a variety of marine organisms, absorb light at one wavelength and emit it at another, longer wavelength; this is, for instance, what gives some jellyfish the ability to glow. As such, they are used by biologists to tag cells through the genetic encoding and in the fusing of proteins.
The researchers found that the fluorophore in these proteins, which enables the immittance of light, can be used as qubits due to their ability to have a metastable triplet state. This is where a molecule absorbs light and transitions into an excited state with two of its highest-energy electrons in a parallel spin. This lasts for a brief period before decaying. In quantum mechanical terms, the molecule is in a superposition of multiple states at once until directly observed or disrupted by an external interference.
Get the world’s most fascinating discoveries delivered straight to your inbox.
To harness this, the researchers developed a custom confocal microscope — a optical system, comprising a series of lenses and mirrors, that uses laser light to produce high-resolution images of biological samples — to optically address the spin state of enhanced yellow fluorescent protein (EYFP) and use it as a qubit in purified protein, a human kidney cell and E.coli bacteria.
The laser microscope initially used a 488-nanometer optical pulse to induce a spin state in the EYFP. A near-infrared laser pulse then triggered a readout of the triplet spin state with "up to 20% spin contrast" — meaning the researchers could see enough differences in spin states to use the protein as a working qubit.
Once the spin has been initialized, the researchers used microwaves to keep the spin in a coherent oscillation between two levels — thus the protein behaved as a qubit for around 16 microseconds before the triplet state decayed.
Biological breakthrough
Observing how the electrons pulse from being hit by a laser means the biological qubit can be used as a quantum sensor, picking up what’s happening inside a cell.
This could yield insight into biological functions at the nanoscale, such as protein folding, the tracking of biochemical reactions in cells and monitoring how drugs bind to target cells and proteins, the scientists said in the study. It could also lead to advancements in medical imaging and the early detection of disease pathways.
While the biological qubit could shake up biological sensing and open up new ways to create quantum sensors, there are hurdles still to overcome.
To effectively manipulate the spin state of the fluorescent protein, it needed to be kept at liquid-nitrogen temperatures. And while the biological qubit proved it could be used effectively in the complex environment of a mammalian cell — a significant part of the breakthrough — it still needed to be cooled to a temperature of 175 kelvin (–98.15 degrees Celsius). At room temperature, this technique still functions in bacterial cells, with the researchers optically detecting magnetic resonance, but only with up to 8% contrast, and with a rapid depletion of the EYFP spin state.
The sensitivity of the biological quantum sensors also lags behind solid-state sensors, such as those made from defects in diamond. So there’s still work to be done on stability and sensitivity before biological qubits and quantum sensors in cells can become practical tools for use in biology and medicine.
But this is a breakthrough that's gone beyond the proof-of-concept stage, and being encoding a qubit directly into a cell opens up a new avenue for quantum technology, where the boundaries between quantum physics and biology are blurred.
Roland Moore-Colyer is a freelance writer for Live Science and managing editor at consumer tech publication TechRadar, running the Mobile Computing vertical. At TechRadar, one of the U.K. and U.S.’ largest consumer technology websites, he focuses on smartphones and tablets. But beyond that, he taps into more than a decade of writing experience to bring people stories that cover electric vehicles (EVs), the evolution and practical use of artificial intelligence (AI), mixed reality products and use cases, and the evolution of computing both on a macro level and from a consumer angle.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus











