New semiconductor could allow classical and quantum computing on the same chip, thanks to superconductivity breakthrough
Researchers believe they can fit 25 million Josephson junctions — a useful component for quantum computing — on one two-inch wafer with this approach.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
In today’s digital age, silicon is king. But as with other semiconductors that are widely used in the industry, trace quantities of other elements are often added to silicon to influence its electronic behaviour, a process known as doping.
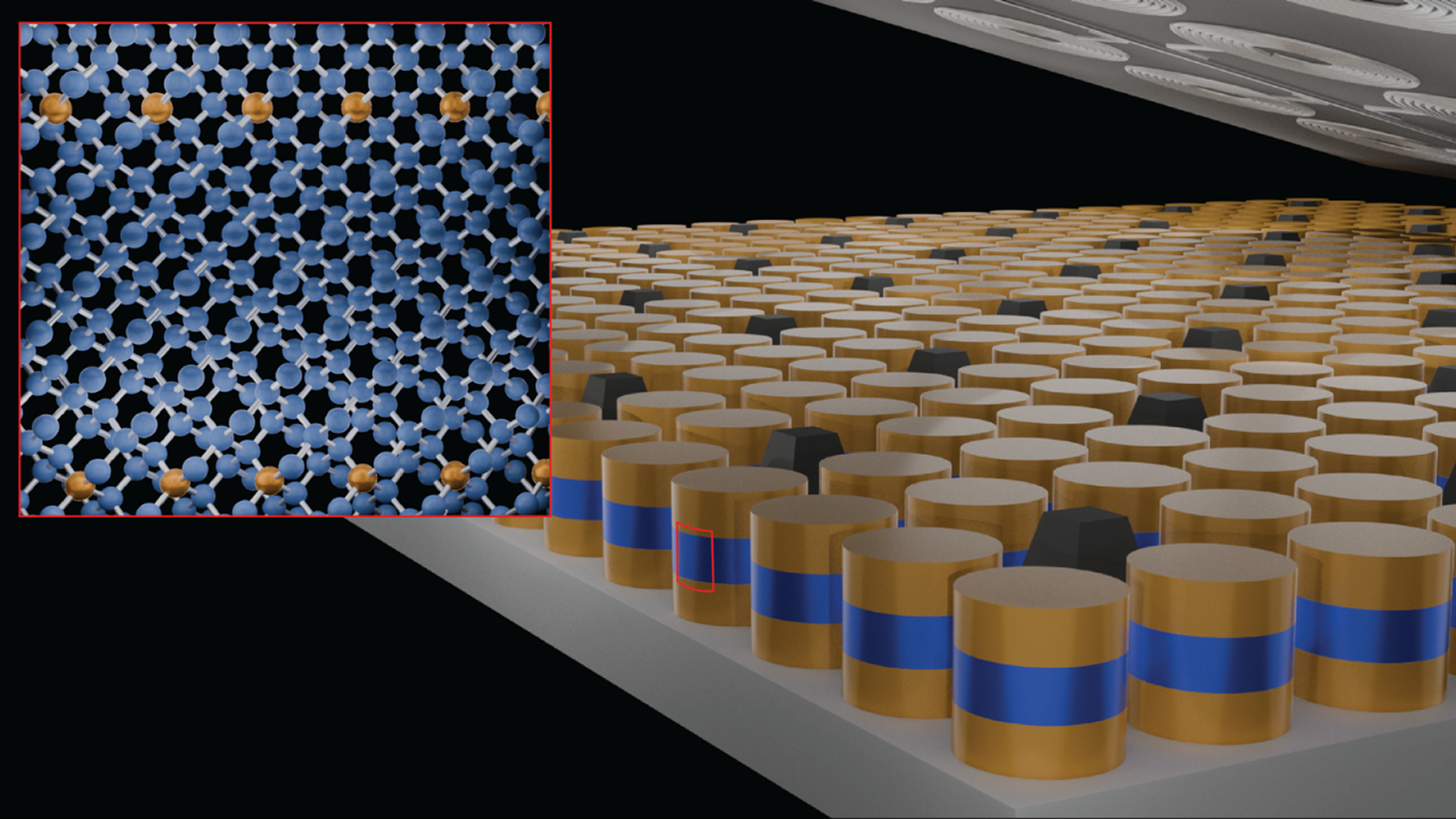
Now, scientists have taken doping to a new level, replacing one in every eight atoms in germanium — a semiconductor similar to silicon – with the superconductor gallium, so that the material forms a new superconductor that can be used for technologies like quantum computing and sensing.
Although silicon is next in line for this approach, germanium is already widely used in industry and is extremely compatible with silicon. The researchers outlined their approach in a new study published Oct. 30 in the journal Nature Nanotechnology.
"I think there is a lot of good reasons to be excited about this," co-author of the study Javad Shabani, a professor of physics at New York University, told Live Science.
The idea of doping a semiconductor enough to render it superconducting was first proposed in 1964 by Marvin Cohen, Professor Emeritus at the University of California, Berkeley, then at the University of Chicago. The idea was resuscitated in the 2000s and 2010s, when several groups attempted to bombard silicon and germanium with superconducting metals to see if they could achieve the theoretically predicted new phase — but they hit problems.
"When you bombard, you kind of ruin the lattice," Shabani explained, adding that you then need to heat it up and "anneal" it to run further experiments for superconducting behaviour, so it is not clear whether dopant atoms have simply formed an island of superconducting material, or whether a new superconducting phase has formed in the bombarded element. He and his team even tried the experiments themselves. "We just added to the puzzle," he told Live Science.
Layer of hope
Progress finally came when they switched to a technique called molecular beam epitaxy. Here they produced the germanium crystal layer by layer, by exposing the surface to germanium atoms with just the right conditions and concentration of gallium atoms for one of the gallium atoms to substitute in for a germanium atom in each unit cell of the crystal.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Shabani suggested they were likely not alone in thinking molecular beam epitaxy might be worth a try. However, attempts had been discouraged by a lot of negative speculation suggesting that doping to the required levels was not physically possible based on assumptions akin to solubility limits. For example, you can keep dissolving more and more sugar in water up to a point, but once you reach the solubility limit, the solution saturates and the sugar will no longer dissolve but remain in solid lumps. Transfer the same arguments to doping and you might think that beyond a certain limit, the dopant will not evenly distribute either but clump together.
But doping by molecular beam epitaxy is a different kind of process altogether — the two materials are laid down together — so it is not limited by anything akin to a solubility limit. "We are just spraying something on something," said Shabani, adding that no laws are violated.
To check what they had, Shabani and his team sent their samples to colleagues at the University of Queensland in Australia to characterize them with their state-of-the-art equipment. As Julian Steele, a researcher at the University of Queensland in Australia who helped with the characterization experiments, pointed out, usually "the precision required" to characterize the interesting superconducting layer buried in the bulk germanium would be experimentally "intractable."
"It was a fortunate combination of well-defined crystal layers and very precise measurements that worked in tandem to produce data with atomic-level precision," Steele told Live Science in an email. "The result is an undeniably clear picture of a new and fascinating quantum material."
The researchers also noted that the superconducting transition temperature was 3.5 Kelvin (just above absolute zero) — cryogenically cold, but not as cold as the 1 Kelvin required to achieve superconductivity in pure gallium. As Shabani highlighted, normally you would expect the transition temperature to be even lower than that of the "parent" superconductor, in this case gallium. This throws some intriguing questions out as to which of the known mechanisms for superconducting behaviour is at play here.
"It is very satisfying to see continued research with successes in the field of superconductivity in doped semiconductors, which I initiated over sixty years ago," Cohen told Live Science in an email. "I believe that there is still much to be learned about superconductivity through research on systems of this kind."
Building more robust qubits
Peter Jacobson, a University of Queensland researcher who also helped with the characterization experiments, was particularly impressed by "how clearly the distortion emerged."
He pointed out that the spacing of the atoms in the plane of each deposited crystal layer remained essentially unchanged from the pure germanium seed layer, but that the spacing perpendicular to this plane increased slightly, just as would be expected to accommodate the slightly larger gallium atoms. "Seeing this behaviour so clearly is a strong indication of how little disorder is present in these films."
That low disorder is good news for anyone seeking to “grow” alternating layers of semiconducting and superconducting material, something which has not been possible before.
This drastically increases the device density that can be achieved on a wafer, because it means you can build up into 3D stacks. Shabani uses the example of a Josephson junction — a junction of a non-superconducting material sandwiched between superconducting material either side. These can be used in quantum sensing and for qubits in quantum computing.
"You can fit 25 million of these on one wafer," he said. He points out that currently each Josephson Junction is around a millimetre in size and added: "Each of these could be a qubit. It could be a pixel of a sensor, right?"
The close adherence to regular crystalline order may have additional benefits for protecting against “decoherence” of superconducting qubits. When qubits decohere, they are no longer capable of holding multiple values at once but lump for a definite value and essentially respond as a classical qubit without the advantage of quantum behaviour.
This is a bugbear in efforts towards quantum computing, but it has been suggested that some of this decoherence may be associated with amorphous characteristics in the materials used. Further experiments will be needed for verification, but the improved crystallinity in these molecular beam epitaxy gallium-doped germanium structures may help qubits to be more robust against decoherence.
What is quite clear is the potential advantage of using the fabrication methods that already exist to make germanium and silicon semiconductor computer processors and devices.
"You have a trillion-dollar silicon germanium infrastructure that now can use superconductivity as a new item in their toolbox,” said Shabani. "That may really help solid-state quantum computing — the timeline could really shrink."

Anna Demming is a freelance science journalist and editor. She has a PhD from King’s College London in physics, specifically nanophotonics and how light interacts with the very small. She began her editorial career working for Nature Publishing Group in Tokyo in 2006. She has since worked as an editor for Physics World and New Scientist. Publications she has contributed to on a freelance basis include The Guardian, New Scientist, Chemistry World, and Physics World, among others. She loves all science generally, but particularly materials science and physics, such as quantum physics and condensed matter.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus










