Widely used epilepsy drugs tied to rare, deadly side effect, FDA warns
The announcement comes after the FDA became aware of more than 40 cases of "DRESS syndrome" linked to the seizure medications levetiracetam and clobazam.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
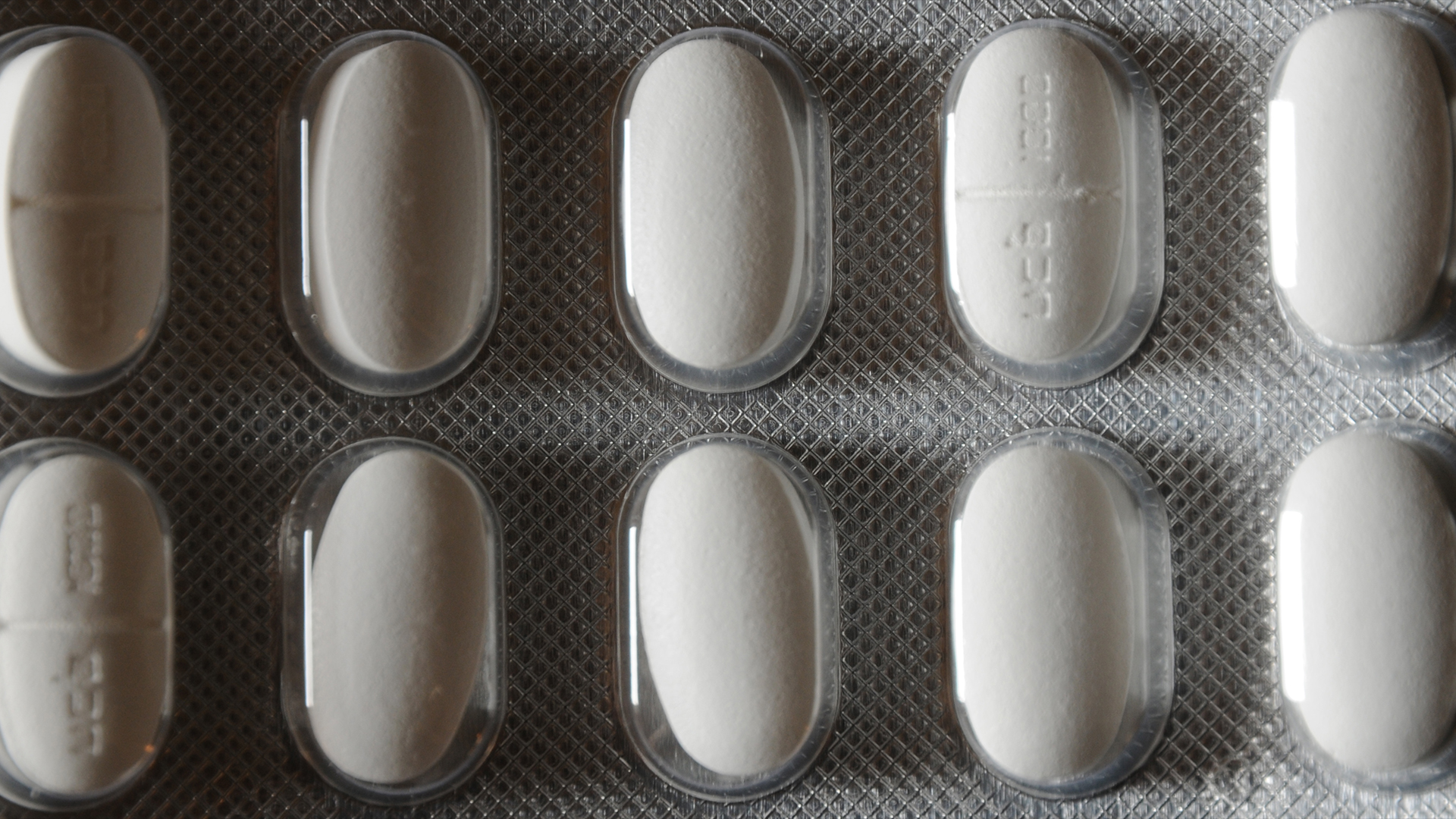
Two widely prescribed epilepsy drugs have been linked to a rare but serious allergic reaction that can be life-threatening, the U.S. Food and Drug Administration (FDA) warns.
Worldwide, more than 40 serious cases of the reaction, known as Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome, have been detected in people taking the drugs levetiracetam and clobazam. The former is more commonly known under brand names such as Keppra or Keppra XR, and the latter is branded as Onfi or Sympazan.
Although the risk of developing DRESS syndrome is rare, the reaction can be deadly if not diagnosed and treated quickly. If anyone taking levetiracetam or clobazam develops any unusual symptoms, such as an unexplained rash, fever or swollen lymph nodes at any time while taking the drugs, they should "go to an emergency room immediately," the FDA said in its announcement on Nov. 28.
While the FDA encourages patients and health care providers to be on the lookout for DRESS, the agency advises that anyone taking levetiracetam or clobazam should not stop taking the medications without first talking to their health care provider. Abruptly stopping these medicines could lead to "uncontrolled seizures," the FDA cautioned.
Related: Teen's year-long case of depression and seizures caused by brain-injuring autoimmune disease
Levetiracetam and clobazam are both widely prescribed in the U.S. for epilepsy, a chronic brain condition that causes recurrent seizures. In 2022, for example, an estimated 12 million levetiracetam prescriptions and almost 800,000 clobazam prescriptions were dispensed from U.S. outpatient pharmacies. Overall, an estimated 1.2% of the U.S. population has active epilepsy, meaning they're currently taking medication to control it and/or they've had at least one seizure in the past year.
Levetiracetam is used to control specific types of seizures associated with epilepsy, including partial seizures, meaning those that affect just one part of the brain, and myoclonic seizures, which cause brief muscle jerks. Clobazam is a depressant that works by slowing down the activity of the central nervous system. It is approved by the FDA to be used alongside other drugs to control seizures associated with a severe form of epilepsy called Lennox-Gastaut syndrome.
Get the world’s most fascinating discoveries delivered straight to your inbox.
To date, the FDA is aware of 32 serious cases of DRESS syndrome in children and adults who had been taking levetiracetam, and 10 cases in people who were on clobazam. The agency learned of these cases after reviewing data reported to the FDA Adverse Event Reporting System (FAERS) database and assessing other published medical literature.
Most of the 42 patients had to go to hospital and receive medical treatment, and two of the patients died.
DRESS syndrome can be triggered by a wide variety of drugs, although it's usually triggered by a single medication in a person's regimen. Symptoms usually begin around two to eight weeks after initial exposure to the drug and may include fever, skin rash, swollen lymph nodes and a swollen face. The rash, which typically looks like flat-to-slightly-raised red spots, is often one of the first symptoms and can spread to the trunk, arms and legs. However, not all patients develop the rash.
DRESS syndrome can also lead to inflammation and injury in various organs, such as the liver, kidneys and heart, which can potentially lead to death.
DRESS syndrome can sometimes be confused with other serious reactions to medication that affect the skin, such as Stevens-Johnson syndrome. However, both syndromes usually require treatment in a hospital.
In light of its announcement, the FDA now requires that manufacturers add warnings about DRESS syndrome to the prescribing information and patient medication guides for levetiracetam and clobazam. The goal is to inform patients and health care providers about the potential risks of the drugs and the early signs of DRESS syndrome.
In light of its announcement, the FDA now requires that manufacturers add warnings about DRESS syndrome to the prescribing information and patient medication guides for levetiracetam and clobazam. The goal is to inform patients and health care providers about the potential risks of the drugs and the early signs of DRESS syndrome.
The agency also urges anyone to report any side effects they experience while taking these drugs to the FDA MedWatch program.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.
 Live Science Plus
Live Science Plus





