Diet change could make brain cancer easier to treat, early study hints
A new lab study exploited a unique aspect of metabolism in glioblastoma to boost the effectiveness of chemoradiation, turning the cancer's properties against itself.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
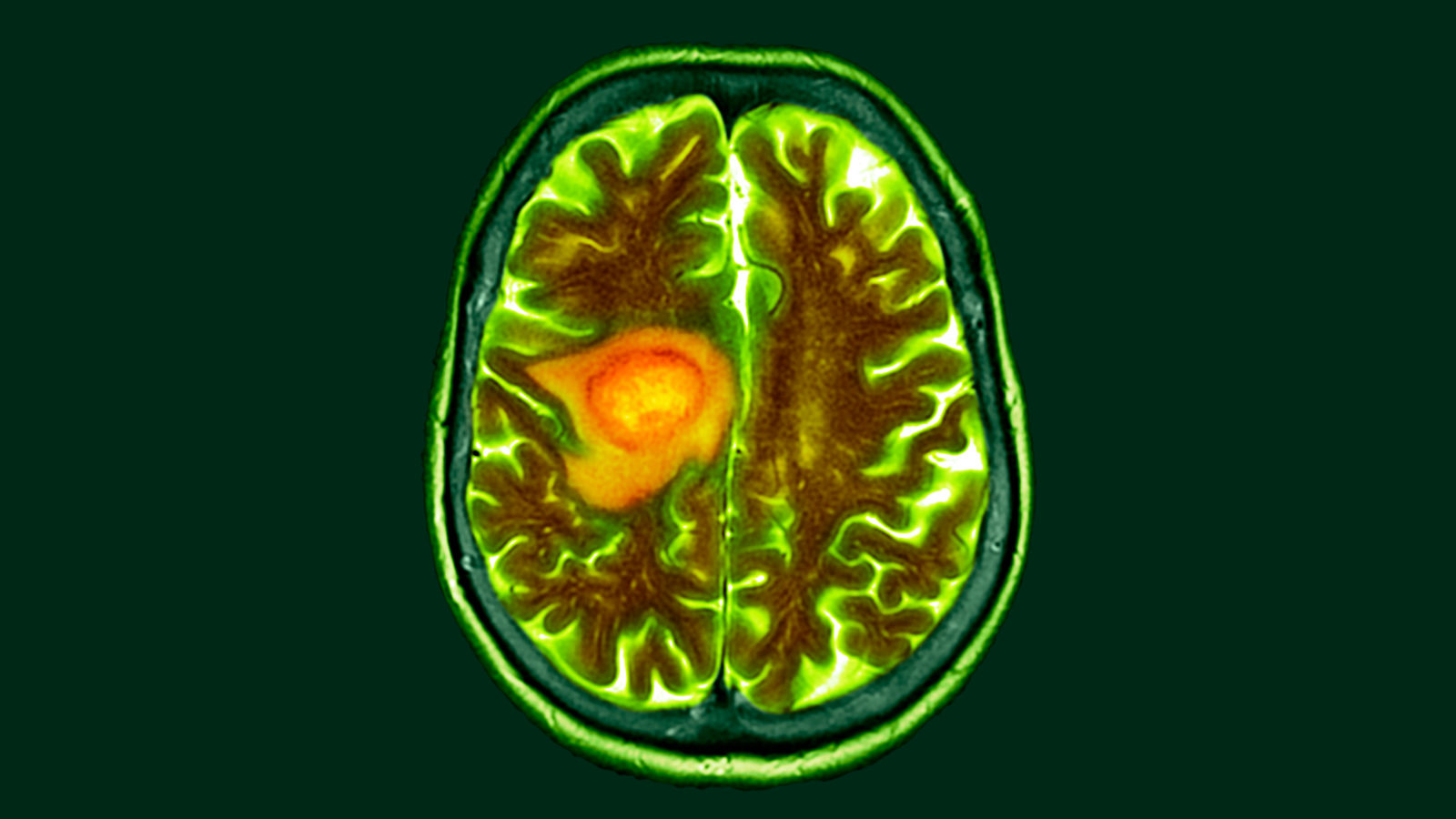
A dietary change may make the fatal brain cancer glioblastoma more vulnerable to cancer therapies, a new study suggests.
The researchers behind the work think this dietary change exploits a key metabolic vulnerability in the cancer, and their work demonstrates that the approach extends survival in mice when used in combination with chemoradiation therapy.
The study, published Sept. 3 in the journal Nature, suggests that a deeper understanding of how tumor cells tweak their metabolisms in the pursuit of all-out growth could open treatment windows for this notoriously deadly cancer.
Healthy cells in the brain need fuel to maintain their extensive list of functions, like electrical signaling and the release of chemical messengers. Cancer cells dispose of these normal processes as they rewire to become "professional dividing cells," said study co-author Costas Lyssiotis, a professor of oncology at the University of Michigan.
These changes are of great interest to cancer researchers because they may allow therapies to distinguish between healthy cells and tumors, making them more targeted.
"The real art of delivering therapy is making it so that you kill the cancer way more than you kill the normal [cells]," Lyssiotis told Live Science.
Related: New treatment for most aggressive brain cancer may help patients live longer
Get the world’s most fascinating discoveries delivered straight to your inbox.
The work, coordinated by University of Michigan oncologist Dr. Dan Wahl, assessed how glioblastoma warps its metabolism in both human and animal brains. The study ambitiously combined laboratory research and clinical practice by drawing some of its data from tissue taken from the brains of patients undergoing cancer surgery. The study required the collaboration of experts in human and rodent brain surgery, metabolic pathways, and molecular analysis.
The protocol began in the hours before surgery. The patients received an infusion of glucose, which was tagged so that it was detectable by molecular analysis techniques. The glucose flowed through their bloodstreams and into both their healthy and tumorous cells.
A common approach for glioblastoma surgery is to remove the tumor and some surrounding brain tissue to minimize the risk of the cancer quickly growing back. The team took blood samples every 30 minutes during surgery and then flash-froze the excised tumor and healthy tissue for analysis.
These extracted cells had metabolized the glucose, and the researchers tracked the molecules' path through the cells. In concert with experiments in mice, the researchers gained a clear view into what tumor cells were doing differently as they gobbled up the sugar.
Healthy cells metabolized glucose for cell processes like respiration, in which sugar and oxygen are converted into fuel for the cell. These cells also converted glucose into an amino acid called serine, a key ingredient for important neurotransmitter molecules.
The tumor cells, by contrast, set these processes aside. Instead, the cancer cells directed glucose to produce nucleotides — the building blocks of DNA. These molecules are vital fuel sources for the tumor cells' endless replication.
Chemoradiation therapies attack cancer by destroying its DNA, but this rerouting gives the cancer cells a steady source of nucleotides with which to repair the damage. The study showed that the tumor cells also scavenged serine from surrounding tissues to further fuel their growth.
Here, Wahl and his team saw an opportunity. They put mice that had been transplanted with human cancer cells on a feeding regimen that drastically reduced their dietary serine. Lyssiotis suggested that this could potentially be replicated in human cancer patients with a low-protein diet supplemented with serine-free protein shakes.
As this cut the amount of serine available for tumor cells, it forced the cancer to reroute its glucose metabolism back into serine production. In turn, this slashed their nucleotide synthesis and made the cells more vulnerable to chemoradiation. Mice given this treatment combination lived longer than mice that were only given chemoradiation.
Lyssiotis explained that this vulnerability would likely work for a limited time, as glioblastoma cells can deftly adapt their metabolisms. In addition, some tumor cells appeared to rely less than others on scavenged serine. "If you can hit that sweet spot, you deprive them of serine, and you come in with the therapies, you get them before they figure out a workaround," he suggested.
Wahl is already beginning work on a follow-up clinical study to back up these results in mice with data from human cancer patients.
"We hope to be bringing that to our patients later this year or early next year," he said. That work will also involve challenges and coordination. "Going through cancer treatment is hard. We're asking people to come up for radiation every day, to take chemotherapy. I think asking someone to also follow a prescribed diet could be tricky," he added.
But the current study has provided valuable information that will inform that future clinical work. "Part of what we're excited about is that this isotope tracing protocol [tracking the tagged glucose] can tell us which tumors are making serine from glucose and which tumors are taking serine up from the environment," Wahl said.
Lyssiotis noted that the paper's pioneering metabolic analysis has identified additional dietary changes that could be explored in future work. Serine modification is the easiest to implement, for the moment. "We think that that's just the tip of the iceberg," he said.
This article is for informational purposes only and is not meant to offer medical or dietary advice.

RJ Mackenzie is an award-nominated science and health journalist. He has degrees in neuroscience from the University of Edinburgh and the University of Cambridge. He became a writer after deciding that the best way of contributing to science would be from behind a keyboard rather than a lab bench. He has reported on everything from brain-interface technology to shape-shifting materials science, and from the rise of predatory conferencing to the importance of newborn-screening programs. He is a former staff writer of Technology Networks.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus










