Atomic Weights Tweaked for 5 Elements
Standard atomic weights, those numbers emblazoned under the elements on the periodic table, were once thought of as unchanging constants of nature.
But researchers have tweaked the atomic weights of five elements — magnesium, bromine, germanium, indium and mercury — in a new table published by the International Union of Pure and Applied Chemistry (IUPAC).
To calculate standard atomic weight, scientists have traditionally averaged the weights of the stable variations of an element known as isotopes. [Image Gallery: Stunning Peek Inside Molecules]
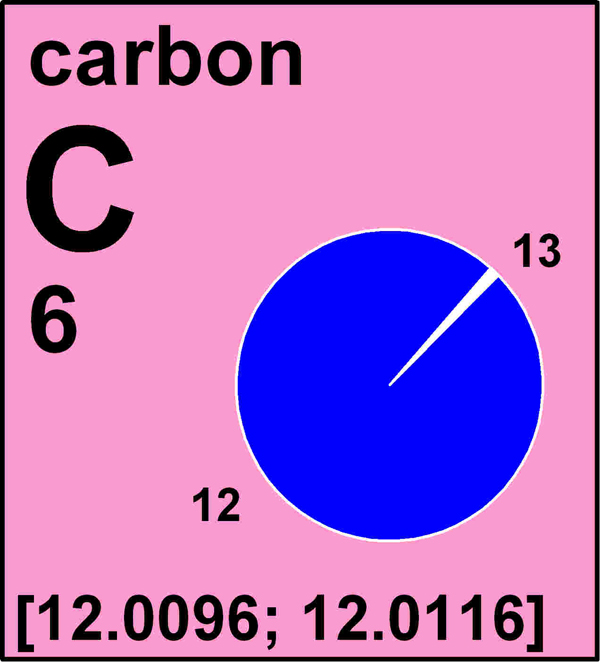
All atoms of an element have the same atomic number, or number of protons in their nuclei, but the number of neutrons in the nuclei can vary, leading some isotopes to be lighter or heavier. Carbon-12, for example, the most abundant carbon isotope, has six protons and six neutrons. Its slightly heavier cousin, carbon-13, has six protons and seven neutrons.
Standard atomic weight also depends on how common an element's stable isotopes are. In other words, the more plentiful an isotope, the more it will influence the average. But the abundance of an isotope can also vary from place to place on Earth, leading to differences in an element's atomic weight depending on its context.
For that reason, the atomic weights of magnesium and bromine will now be expressed as intervals with upper and lower bounds instead of single values. The atomic weight of bromine, for instance, is commonly thought to be 79.904, but it can actually range between 79.901 and 79.907, depending on where the element is found.
For germanium, indium and mercury, improved standard atomic weights were determined through better measurements. The weight of the rare metal indium, for example, is being adjusted from 114.818(3) to 114.818(1), based on a new calculations with a mass spectrometer, a sensitive instrument that can measure the minuscule weight and and relative concentrations of atoms and molecules. (The numbers in parentheses represent the uncertainty in the last digit of the atomic weight.)
Get the world’s most fascinating discoveries delivered straight to your inbox.
The changes aren't unprecedented. In 2010, the IUPAC replaced standard atomic weights with intervals for hydrogen, lithium, boron, carbon, nitrogen, oxygen, silicon, sulfur, chlorine and thallium. Elements like fluorine, aluminum, sodium and gold have just one stable isotope and thus do not exhibit variations in their atomic weights.
The new intervals may cause some confusion for chemistry students trying to decide which atomic-weight values to use when making precise calculations, said Ty Coplen, director of the U.S. Geological Survey's Stable Isotope Laboratory in Reston, Va., who contributed to the research that led to the new atomic weights.
"For more than a century and a half, many students have been taught to use standard atomic weights — a single value — found on the inside cover of chemistry textbooks and on the periodic table of the elements," Coplen said in a statement. "Though this change offers significant benefits in the understanding of chemistry, one can imagine the challenge now to educators and students who will have to select a single value out of an interval when doing chemistry calculations."
Such minutiae can have practical implications. The abundances of carbon isotopes can be used to investigate the purity of the source of foods like vanilla and honey, while isotopic measurements of elements like nitrogen and chlorine can help scientists tracing pollutants in streams and groundwater, according to a statement from the USGS.
The report is detailed in the journal Pure and Applied Chemistry.
Follow Megan Gannon on Twitter and Google+. Follow us @livescience, Facebook & Google+. Original article on LiveScience.com.

 Live Science Plus
Live Science Plus