Facts About Technetium
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
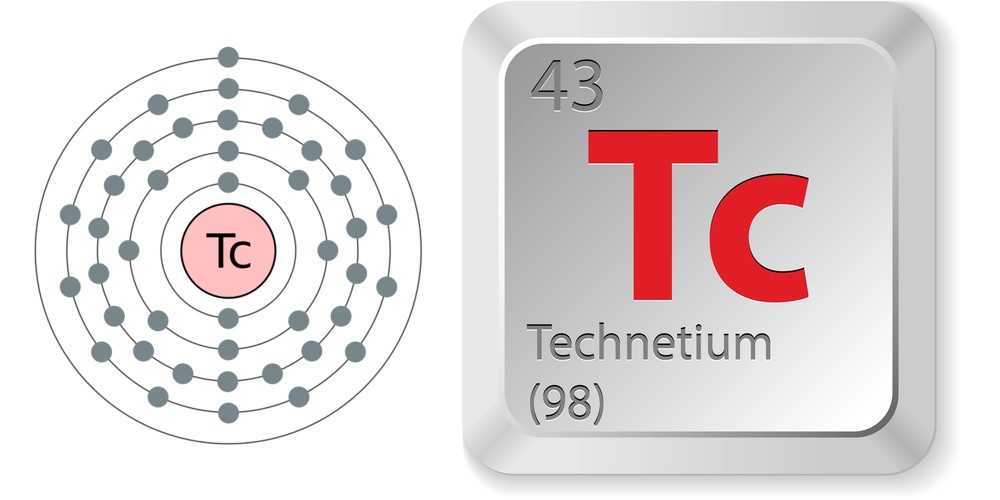
| Atomic Number: 43 Atomic Symbol: Tc Atomic Weight: 98 | Melting Point: 3,915 F (2,157 C) Boiling Point: 7,709 F (4,265 C) |
Word origin: The word technetium comes from the Greek technetos, artificial.
Discovery: Technetium was the first element to be produced artificially. Element 43 was predicted on the basis of a gap in the Periodic Table of the Elements, and in 1925 its discovery was erroneously reported. At that time, it was named masurium.
Technetium was actually discovered — produced artificially — in 1937 by Perrier and Segre in Italy. It was also found in a sample of molybdenum that was bombarded by deuterons in a cyclotron. Since then, scientists have searched for it in terrestrial material. None was found until 1962, when isotope technetium-99 (99Tc) was isolated and identified in African uranium ore in extremely tiny quantities. It was a spontaneous fission product of uranium.
Properties of technetium
Technetium is a silvery-gray transition metal. It tarnishes slowly in moist air. It dissolves in nitric acid, aqua regia (nitro-hydrochloric acid) and concentrated sulfuric acid, but is not soluble in any strength of hydrochloric acid.
Technetium’s common oxidation states are +7, +5, and +4. Under oxidizing conditions technetium (VII) will exist as the pertechnetate ion, TcO4-.
Technetium can be chemically bound to many biologically active molecules.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Every form of technetium is radioactive.
Sources of technetium
Technetium is primarily obtained artificially.
If technetium does exist organically, it must be in minute concentrations. Technetium has been found in the spectrum of S-, M-, and N-type stars. Its presence is leading to new theories of the production of heavy elements in the stars.
As was found in 1962, one of its isotopes, 99Tc, is produced as a product from the fission of uranium in nuclear reactors. Because of this, large quantities have been produced over the years. There are kilogram-sized quantities of technetium currently in existence. 99Tc is a contamination hazard and should be handled in a glove box.
Uses of technetium
Technetium is a remarkable corrosion inhibitor for steel. Mild carbon steels may be protected by as little as 55 ppm of KTcO4 in aerated distilled water at temperatures up to 250 C (482 F). This corrosion protection is limited to closed systems, since technetium is radioactive and must be confined.
It is also an excellent superconductor at 11K (-439.9 F / -262.1 C) and below.
One of technetium’s long-lived isotopes,95Tcm ("m" stands for meta state) (T1/2 = 61 days), is used in tracer work. The element’s most useful isotope, however, is 99Tcm (T1/2 = 6.01 hours). It is used in many medical radioactive isotope tests because of its short half-life, gamma-rays’ energy, and technetium’s ability to be chemically bound to many biologically active molecules.
Isotopes of technetium
There are 22 reported isotopes of technetium, all of them radioactive. It is one of only two elements with Z < 83 that have no stable isotopes (the other being promethium). Its isotopes range from 90 to 111.
Technetium has three long-lived radioactive isotopes: 97Tc (T1/2 = 2.6 x 106 years), 98Tc (T1/2 = 4.2 x 106 years) and 99Tc (T1/2 = 2.1 x 105 years).
(Source: Los Alamos National Laboratory)
 Live Science Plus
Live Science Plus