Animal Code: Our Favorite Genomes
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Why we sequence genomes
Every part of our bodies and every action of our cells is exquisitely controlled by the billion base pairs that make up our DNA. These nucleotides are the building blocks of genes, the part of the genome that holds the information our cells turn into proteins.
Since the first gene was suggested by Gregor Mendel in the 1860s, scientists have been searching for ways to decode them, to figure out how this code creates the end product: An organism. That organism can be an animal, plant, virus or bacteria that lives, reproduces and spreads its genome. Uncovering the secrets locked in each species' genomes will teach researchers how to harness the power of genes, from the longevity of the naked mole rate and the fat processing abilities of the orangutan.
Dozens of animal, plant and microbe genomes have been sequenced. Here are LiveScience's favorite 10 genome projects.
Culturing cow

Itching for a tastier slice of beef? Look no further than the cow's genome. After being sequenced in 2009, analysis of the cow's genes could lead to higher quality milk and better beef and tells an interesting tale of how human domestication has impacted the evolution of the once-wild animal.
The analysis of the cow's 22,000 genes has also shown that while we humans are more closely related to rodents than to cows in terms of the family tree, our genome more closely resembles those of cows because the tiny lifespan of rodents requires them to have many more babies in a much quicker timeframe, accelerating evolution.
The cows also have many additional immune system genes, ones that could defend against pathogens that live in their extra stomachs. Analysis of other breeds also determined that the cows showed specific patterns of genetic changes depending on whether they were breed for meat or milk.
First amphibian, the African clawed frog

The first amphibian genome to be sequenced belongs to the frog Xenopus tropicalis, a slimy rotund amphibian also known as the African clawed frog. The genome study enables researchers to compare genes in mammals to those of the amphibians to see which genes stay the same and which have changed since mammals and amphibians parted 360 million years ago, which pinpoints the important basic genes that all complex life needs, including genes involved in the heart and lungs.
Get the world’s most fascinating discoveries delivered straight to your inbox.
The tasty turkey

The turkey genome was published in the journal PLoS One in November of 2010, just in time for the Thanksgiving meal! The turkey clocked in at 1.1 billion base pairs, about a third the size of the human genome, and bears a close resemblance to its relative, the chicken, whose genome was completed in 2004.
This work could lead to meatier, healthier birds, according to the researchers, by providing a better understanding of the turkey's muscles and taste and can help farmers improve disease resistance and treatment.
Our cousin the orangutan

A study in the journal Nature in 2011 released the genes of the orangutan Susie (and five of her wild brethren) of the Texas Zoo. The genes revealed that orangutans have been evolving much more slowly than chimps and humans; their genes change around much less frequently. This could mean that chimps and humans have accelerated their evolution since separating from the rest of the primates.
The orangutans were evolving in one respect: Their fat breakdown molecules were changing quicker than expected. This is probably why they make better use of the energy they take in.
Spiny, spineless sea urchin

Humanity's evolutionary cousin, the spiny but spineless sea urchin, received the honor of having its genome sequenced in 2006 and published in the journal Science. Seventy percent of the urchins' 23,300 genes (made from 814 million pairs of genetic bases) are similar to those in humans, more than many other lab organisms like fruit flies.
The urchins' genes also hold details where the urchins get their unique immune system, and the secrets of their 100-year-long lives. The genes of the innate immune system, our body's first line of defense, multiplied in the urchin, giving them a larger toolbox to combat infections.
Though they lack eyes and ears, the researchers discovered that the urchins sport genes associated with vision and hearing as well as taste, smell and even balance.
Rhesus monkey

The first primate to get rocketed into space and to be cloned, the rhesus monkey, had its genome sequenced in 2007. The rhesus monkey genome has about 93 percent similarity with that of humans, which is important since it is often used in medical testing for human drugs and therapies.
The researchers identified roughly 200 genes that appear to be key players in what defines the differences between our species. These include genes involved in hair formation, sperm-egg fusion, immune response and changes to cell membrane proteins.
The rhesus monkey shows the same mysterious rearrangements that are seen in the human lineage's X chromosome following the branching off of the chimpanzee which gives us new evidence of the unusual role of this sex chromosome in primate evolution.
Marsupials versus mammals

Marsupials, our mammalian brethren, are found mostly in Australia and New Guinea. They have many weird features that separate them from other mammals, including a very short pregnancy, after which they shelter their very immature offspring in a pouch.
Sequences of the kangaroo and other marsupials have shed light on how these features have developed after the placental mammal-marsupial split 150 million years ago. The genome sequencing of an opossum and a small kangaroo species called the tammar wallaby show that the group may have evolved in South America, not Australia.
Analysis of the tammar wallaby genome indicates that large areas of the marsupial genome are similar to the genome of normal, placental mammals.
Nematodes
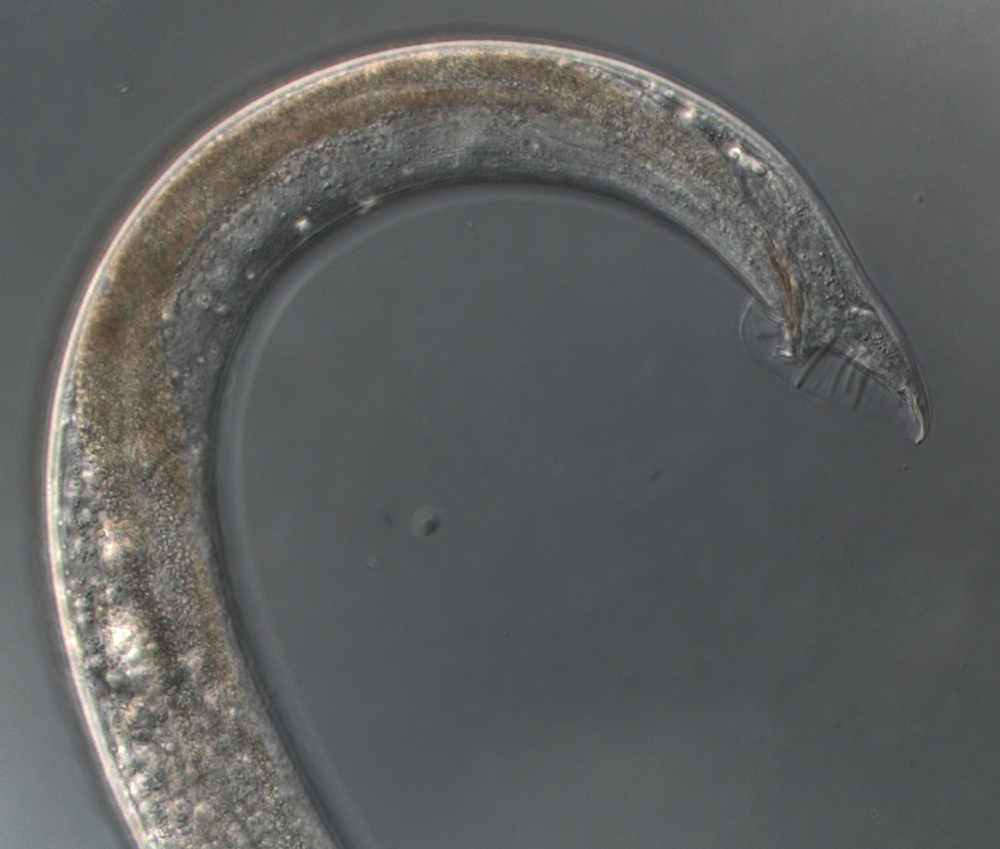
One of the first multi-cellular organisms to have its genome decoded, way back in 1998, the nematode is a staple in many research labs. The nematode and its simple-minded cousins have about 20,000 genes. While similar in number to those of other animals, the nematode's genome contains only 100 million base pairs of DNA; one tenth the size of an average mammalian genome.
This is because more evolved organisms tend to have more non protein coding regions that regulate how, when and how much of a gene is expressed in different types of cells, and not necessarily have more genes. This fine-tuned regulation seems to play an important part in what makes mammals and other organisms unique.
Human
The first human genome was sequenced in 2001, and currently over 60 complete human genomes have been sequenced. These include the genome of researcher J Craig Venter, James Watson (who helped discover the double helix shape of DNA), a Han Chinese, a Yoruban Nigerian, a female leukemia patient and a Korean individual.
Comparison of these genomes to the genome of the chimpanzee and other organisms and looked at which seem to disappear in humans. These genes are likely to play an important role in what makes us humans, though only one held the code for an actual protein, the rest were involved in regulation or had other functions.
They found differences in the handling of several proteins, including ones in the brain, and ones that respond to male hormones. These changes resulted in bigger brains and changes in penis shape in response to a decrease in polyandry in humans. (Many other species have specially shaped penises, sometimes with spines, to compete with the sperm of other males.)
The enigmatic naked mole rat

The newly deciphered genome of the hairless, underground-dwelling, long-lived and cancer-resistant naked mole rat could help researchers unravel the creature's secrets, and may help improve human health along the way.
The researchers found that the naked mole rat had turned off several genes related to vision since they live in the dark. They also saw a mutation in the gene dubbed "hairless," previously seen to cause baldness in mice and humans, which could explain how they lost their fur.
While a quick cursory look at the genome sequencing is already shedding light on changes that may lead to the naked mole rat's exquisite uniqueness, the information is also useful for human health. Stroke and heart attack deprive parts of the body from oxygen. Discovering how the mole rats survive in their low-oxygen burrows can help scientists design treatments to improve outcomes.
By comparing this genome with those of other mole rats, including solitary ones, scientists could also tease out how the animal's genes influence their behaviors.
Jennifer Welsh is a Connecticut-based science writer and editor and a regular contributor to Live Science. She also has several years of bench work in cancer research and anti-viral drug discovery under her belt. She has previously written for Science News, VerywellHealth, The Scientist, Discover Magazine, WIRED Science, and Business Insider.
 Live Science Plus
Live Science Plus











