Facts About Nickel

Nickel is a hard, silvery-white metal whose strength, ductility and resistance to heat and corrosion make it extremely useful for the development of a wide variety of materials — from wires to coins to military equipment.
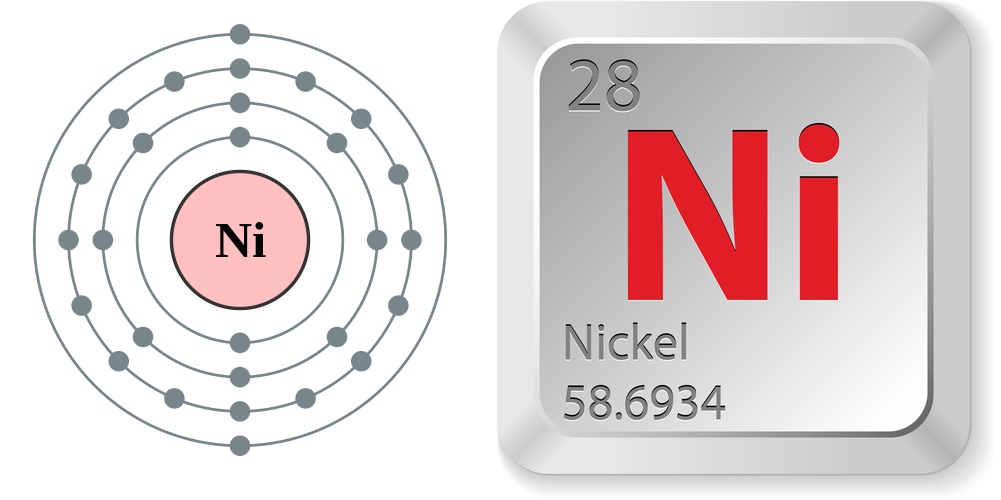
This extremely useful metal is No. 28 in the periodic table of the elements, between the elements cobalt and copper. Nickel is a fairly good conductor of electricity and heat and is one of only four elements (cobalt, iron, nickel and gadolinium) that are ferromagnetic (magnetized easily) at room temperature. Nickel is a transition metal, meaning it has valence electrons in two shells instead of one, allowing it to form several different oxidation states.
Discovery
The discovery of nickel ore in 17th-century Europe is a tale of mistaken identity and superstition. In the 1600s, German miners searching for copper in the Ore Mountains came upon a previously unknown nickel ore (known today as nickel arsenide or niccolite) — a pale brownish-red rock of nickel and arsenic. Believing they'd discovered another copper ore, the miners attempted to extract the copper, but, of course, the rocks failed to produce. The frustrated miners blamed Nickel, a mischievous demon in German mythology, for playing a prank on them and began calling the ore kupfernickel, translated as "copper demon."
About a century later, in 1751, the Swedish alchemist Baron Axel Fredrik Cronstedt tried heating kupfernickel with charcoal and found that its various properties — such as being white and magnetic — clearly revealed that it wasn't copper. Cronstedt is credited as the first person to extract nickel and isolate it as a new element. He dropped the name "kupfer" and called the new element nickel.
Where on Earth?
Nickel is the fifth most abundant element on Earth. However, it is 100 times more concentrated below the Earth's crust than in it, according to Chemicool. In fact, nickel is believed to be the second most abundant element in the Earth's inner core, with iron being the first by a large margin.
Nickel is typically found in two types of deposits: laterite deposits, which are the result of intensive weathering of surface nickel-rich rocks, and magmatic sulfide deposits. Nickel can also be found in manganese nodules and crusts on the deep sea floor, but currently these are not being mined, according to Geology.com. The main mineral sources of nickel are limonite, garnierite and pentlandite.
In 1848, Norway became the first large-scale nickel smelting site. Here they used a type of nickel ore known as pyrrhotite. In 1883, large deposits of nickel were discovered in the Sudbury Basin in Canada, which is where about 30 percent of the world's nickel is mined today. It is believed that this large deposit of nickel is due to an ancient meteor impact. More nickel was discovered in Russia and South Africa in the early 1900s, further allowing nickel to take a strong place in industry.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Just the facts
- Atomic number (number of protons in the nucleus): 28
- Atomic symbol (on the periodic table of the elements): Ni
- Atomic weight (average mass of the atom): 58.6934
- Density: 8.912 grams per cubic centimeter
- Phase at room temperature: solid
- Melting point: 2,651 degrees Fahrenheit (1,455 degrees Celsius)
- Boiling point: 5,275.4 F (2,913 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 5 stable; 26 unstable
- Most common isotope: NI-58 (68.077 percent natural abundance)
Super metal
Few things are made of pure nickel. Instead, nickel tends to play a supportive and stabilizing role in industry materials; it is usually combined with other metals to produce stronger, shinier and more durable products. Nickel is commonly used as a protective outer coating for softer metals.
Because of its ability to withstand extremely high temperatures, nickel is the metal of choice for making superalloys — metal combos that are known for great strength as well as resistance to heat, corrosion and oxidation. In fact, approximately 65 percent of nickel is used to manufacture stainless steels and another 20 percent is used to make other steel and non-iron alloys, including highly-specialized military, aerospace and industrial uses, according to the Nickel Institute. About 9 percent is used in plating and 6 percent goes toward other types of applications, such as coins, batteries and electronics.
Money talks
Since nickel is such a sturdy and corrosion-resistant material, it is an excellent metal for coin-making. The first coin to include the metal nickel was the one-cent piece Flying Eagle, an American coin made of 12 percent nickel and 88 percent copper. This coin, which was also the first to go by the name "nickel," only circulated two years, between 1857-1858.
The five-cent nickel was introduced a few years later in 1866 just after the Civil War came to an end. Prior to the war, most coins were still made of gold and silver, which gave them intrinsic value. As rumors of war began to spread, people started hoarding these valuable coins, causing the economy to take a nosedive. To compensate for the lack of coins, people started trading stamps and notes that had been issued by banks and shops. Even Union soldiers were being paid with notes by the government.
In the meantime, industrialist Joseph Wharton had bought several nickel mines in the early years of the war. Nickel was a much-needed metal during the war as it was used to help make military equipment. Once the war ended, however, there was a surplus of nickel, and Wharton probably wondered what he was going to do with all of his nickel. He urged legislators to start making more coins with nickel. After all, people would be a lot less likely to hoard this less valuable metal.
The lawmakers listened, and in 1866, the American government minted 15 million 5-cent coins made of 75 percent copper and 25 percent nickel. Nickels flooded the economy and became the most prominent coin in the after-war years.
In the United States, nickels, dimes, quarters and half-dollars are still made of a copper-nickel alloy. The nickel coin, quite aptly, contains the most nickel at 25 percent, while the other coins are 8.33 percent nickel.
Who knew?
- Many meteorites consist of a nickel-iron alloy. People in Ancient Egypt considered these nickel-rich meteorites so significant that they shaped them into objects of beauty. Tube-shaped beads made of meteorite material (about 30 percent nickel weight) were discovered in grave pits in Gerzeh cemetery on the west bank of the Nile River. It is believed they were buried around 3300 B.C.
- Nickel is used as a catalyst for the hydrogenation of unsaturated compounds in several types of oils, changing substances from liquids to solids. A nickel catalyst may be used to produce margarine, shortening and certain types of soap.
- Nitinol is a nickel alloy that remembers its previous shape, according to Chemicool. If you heat a wire of this nickel-titanium alloy at 500 C (932 F), you can bend the wire into any shape you'd like. Allow it to cool and bend it into another shape. Then reheat the wire — and watch the first shape reappear.
- The explosion of Supernova 2007bi created a mass of nickel three times greater than our sun, which caused the expanding gases to glow very brightly for months.
- Nickel allergy is one of the most common causes of allergic contact dermatitis. A common irritant is jewelry that contains nickel, such as white gold.
- Switzerland produced the first coin of nearly pure nickel in 1881, and Canada — the world's biggest nickel producer — also introduced a 99.9 percent nickel coin worth five cents between 1922 and 1981.
- Adding nickel to glass gives it a green color.
- A single kilogram of nickel can be drawn into 300 kilometers of wire, according to the Jefferson Lab.
Magnetism
Nickel is one of only four metals that are ferromagnetic, meaning they are attracted to magnets and are magnetic themselves. The others are iron, cobalt and gadolinium. Alnico magnets — a combination of aluminum (Al), nickel (Ni) and cobalt (Co) — are very strong permanent magnets that retain their magnetism even when heated until they glow red, according to Chemicool.
Mu-metal is a soft magnetic alloy of approximately 80 percent nickel and 20 percent iron (and a dash of molybdenum). Mu-metal has very high permeability, which allows it to shield sensitive electronic equipment against static or low-frequency magnetic fields. If you place Mu-metal between a magnet and metal, the usual attraction actually disappears.
Toxicity
Nickel is an essential element for healthy plant life, and trace amounts are naturally found in most vegetables, fruits, nuts and in slightly greater amounts in chocolate and wine, according to the Nickel Institute.
But like most metals, nickel has a dark side when too much enters the human body. When large amounts of nickel — which is a known carcinogenic — accumulate in the soil, air, or our food and water supply, there are risks of toxicity.
People who work in jobs of nickel refining, electroplating and welding are at the greatest risk for health problems. Workers who breathe in traces of nickel dust are at an increased risk for lung cancer, fibrosis and other ailments.
In 2012, 25 nickel-plating workers in Egypt were given liver function tests. The results overwhelmingly showed poor liver function among the nickel workers compared to the control group. Another study in Saudi Arabia also exposed the toxic effects of nickel exposure on the lungs and airway, but the researchers also discovered an interesting fact: ingesting dietary curcumin, the active ingredient in the spice turmeric, was linked to a significant reduction in toxicity and oxidative stress.
Additional resources