Facts About Beryllium

Uniquely strong and light, beryllium is used to make cell phones, missiles and aircrafts. But workers who handle the metal need to watch out, as airborne beryllium has been known to be highly toxic.
Named after beryllos, the Greek name for the mineral beryl, the element was originally known as glucinium — from Greek glykys, meaning "sweet" — to reflect its characteristic taste. But the chemists who discovered this unique property of beryllium also found that it is in fact highly toxic and should therefore never be tasted, according to Jefferson Lab. In fact, the metal, its alloys and salts should only be handled in accordance with specific work codes. Beryllium is also classified as a carcinogen by the International Agency for Research on Cancer and it can cause lung cancer in people who get exposed to beryllium on a daily basis because of their occupations that require them to mine or process the metal, said Dr. Lew Pepper, a medical researcher at Queens College Center for the Biology of Natural Systems in New York.
Despite its toxicity, the element is highly useful because of its unique qualities. For instance, it is one of the lightest metals and has one of the highest melting points among the light metals, according to the Los Alamos National Laboratory. Steel gray in color, beryllium's modulus of elasticity is about one-third greater than steel. Beryllium is nonmagnetic and resistant to concentrated nitric acid. It also has superior thermal conductivity and resists oxidation in air in normal temperatures.
Just the facts
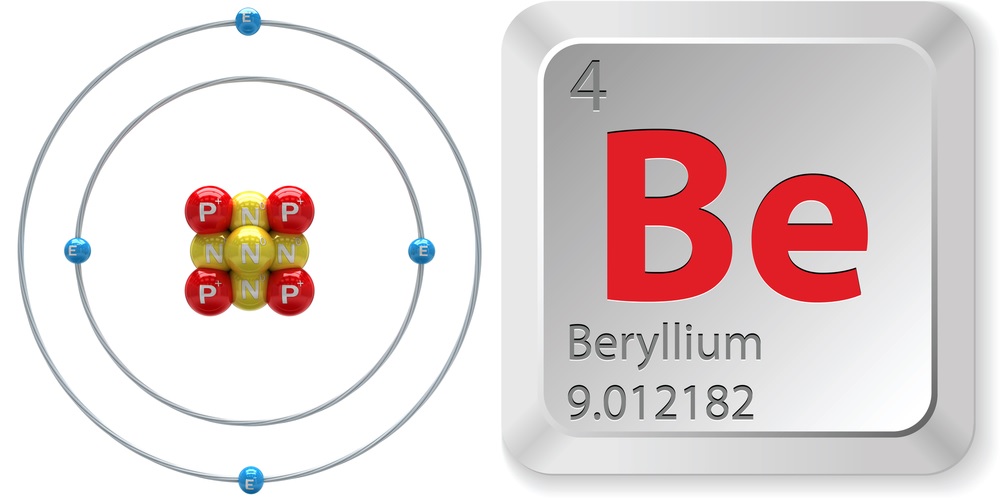
- Atomic number (number of protons in the nucleus): 4
- Atomic symbol (on the Periodic Table of the Elements): Be
- Atomic weight (average mass of the atom): 9.012182
- Density: 1.85 grams per cubic centimeter
- Phase at room temperature: Solid
- Melting point: 2,348.6 degrees Fahrenheit (1,287 degrees Celsius)
- Boiling point: 4,479.8 F (2,471 C)
- Number of isotopes (atoms of the same element with a different number of neutrons): 12, including one stable isotope.
- Most common isotopes: 9Be (Natural abundance: 100 percent)
- Beryllium discovery and use
Beryllium was discovered in 1798 by the French chemist Louis Nicolas Vauquelin, who found it in the oxide form in beryl and a green-colored variety of beryl, emerald.The metal was isolated in 1828 by two chemists, Friedrich Wölhler from Germany and Antoine Bussy from France, who independently reduced beryllium chloride (BeCl2) with potassium in a platinum crucible, according to the Jefferson Lab. These days, beryllium is typically obtained from the minerals beryl and bertrandite in a chemical process or through the electrolysis of a mixture of molten beryllium chloride and sodium chloride, the Jefferson Lab reports.
Beryllium is found in about 30 mineral species, including bertrandite, beryl, chrysoberyl, and phenacite, according to the Los Alamos National Laboratory. Beryl and bertrandite are the most important commercial sources of the element and its compounds.
Beryllium is alloyed with copper or nickel to make springs, gyroscopes, electrical contacts, spot-welding electrodes and non-sparking tools, according to the Royal Society of Chemistry. Other beryllium alloys are used in high-speed aircrafts and missiles, as well as spacecraft and communication satellites. Beryllium copper is also used in windshield frame, brake discs, support beams, and other structural components of the space shuttle.
Thanks to its low thermal neutron absorption cross-section, beryllium is used in nuclear reactors as a reflector or moderator. Moreover, the high melting point of beryllium oxide makes it a useful material for nuclear work and ceramic applications, according to the Los Alamos National Laboratory.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Who knew?
- Emerald, morganite and aquamarine are precious forms of beryl. Some of the oldest emerald mines were developed by the Romans in the Eastern Desert of Egypt about 2,000 years ago, according to the U.S. Geological Survey.
- Beryllium-containing materials are used in cell phones, other portable devices and cameras, according to the Beryllium Science & Technology Association.
- Beryllium is also present in parts of the analytical equipment used to test blood for HIV and other diseases, the Beryllium Science & Technology Association reports.
- Beryllium played a role in the discovery of the neutron when James Chadwick bombarded beryllium with alpha particles and discovered the subatomic particle with no net electric charge.
- Beryllium was the primary ingredient used to make mirrors in NASA’s James Webb Space Telescope, according to NASA.
- The European Commission lists beryllium as one of the 20 critical raw materials for the European Union.
- The U.S. Department of Defense classifies beryllium as a strategic and critical material as it can be found in products that are essential to national security, according to the U.S. Geological Survey.
- The United States is the world’s leading beryllium source and producer. In fact, just one mine at Spor Mountain, Utah, was the source of more than 85 percent of beryllium excavated worldwide in 2010, the USGS reports.
- Beryllium is highly transparent to X-rays and is therefore used in windows for X-ray tubes, according to Jefferson Lab.
- Louis Nicolas Vauquelin — the chemist who discovered beryllium also discovered another element — chromium.
Current research
Beryllium has long been a topic of interest to researchers who have examined its harmful effects on health in people who are exposed to the metal on a daily basis for long periods of time. However, this risk does not apply to people in the general population who do not actually handle beryllium, Pepper said.
"Beryllium becomes harmful when it is generated into an airborne particulate … which is inhaled and descends into the lungs, and it can have an immunologic response in the individual," Pepper told Live Science. This immunologic response, called beryllium sensitization, can then develop into chronic beryllium disease, which causes scarring of the lung tissue and can be deadly. There is currently no cure for the disease, whose progression can be slowed by medication, oxygen therapy, and lung transplants in severe cases, according to the University of California San Francisco Medical Center.
Interestingly, not all those who get exposed to potentially harmful levels of beryllium will experience an allergic, potentially deadly reaction. "There is a genetic susceptibility component, which means that not everyone who is exposed is at risk of going on to develop sensitization, then chronic beryllium disease," Pepper said. "For the most part, it depends upon a genetic susceptibility," he said.
About 85 percent of people who develop chronic beryllium disease after getting exposed have an immune system protein known as HLA-DP2, according to recent research published in July 2014 in the journal Cell. In the study, researchers determined that the bodies of people who have this protein create a unique molecular "pocket," which captures beryllium ions and triggers an inflammatory response in the lungs.
"The immune system does not actually 'see' beryllium," study author John Kappler, a researcher at National Jewish Health, said in a statement. "The beryllium changes the shape of otherwise innocuous self-peptides [peptides produced by the body's own tissues] so that T cells recognize them as foreign and dangerous." (T cells are white blood cells that are essential for one's immunity.) He added that the new findings could one day lead to new therapeutic strategies to treat and prevent chronic beryllium disease.
Dating geological events with beryllium
Using a technique known as cosmogenic nuclide dating, scientists can determine how long rocks have been exposed to the air by measuring their levels of beryllium-10, a radioactive isotope of beryllium. Cosmogenic nuclide dating is often used to determine the dates of important geological events, such as glacier advances and retreats, rock slides, meteor impacts and lava flows.
For example, after a rock avalanche, boulders that land on top of the heap have surfaces that are exposed to the sky for the first time. Particles from incoming cosmic rays (high-energy radiation from outer space) begin to hit these boulder surfaces, creating beryllium-10. The longer the surface area is exposed, the greater the amount of beryllium-10, according to the University of Utah.
In a recent study, researchers at the University of Utah conducted the first rigorous analysis to date a landslide that had occurred thousands of years ago in what is now Zion National Park in Utah. Scientists have known for some time that the flat floor of this park was previously a lake originally created when a massive rock avalanche dammed up the Virgin River, but it was still unclear exactly when this landslide occurred. To find out, the researchers analyzed the levels of beryllium-10 in 12 boulders taken from the area.
Their findings conclude that the rock avalanche occurred about 4,800 years ago as a single event, with a range of uncertainty that gives or takes 400 years, according to the University of Utah. Their work was published in 2016 in GSA Today, the journal of the Geological Society of America.
Additional reporting by Traci Pedersen, Live Science contributor. Follow Live Science @livescience, Facebook & Google+.
Additional resources
- Here is more info on beryllium from the Los Alamos National Laboratory.
- This video shows how beryllium is extracted in the West Desert of Utah.
- Here is also a series of practical videos on beryllium worker safety training.
 Live Science Plus
Live Science Plus