Scientists discover enzyme that can turn air into energy, unlocking potential new energy source
A relative of the tuberculosis bacterium has long been known to convert hydrogen from the air into electricity. Now, scientists have discovered how.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
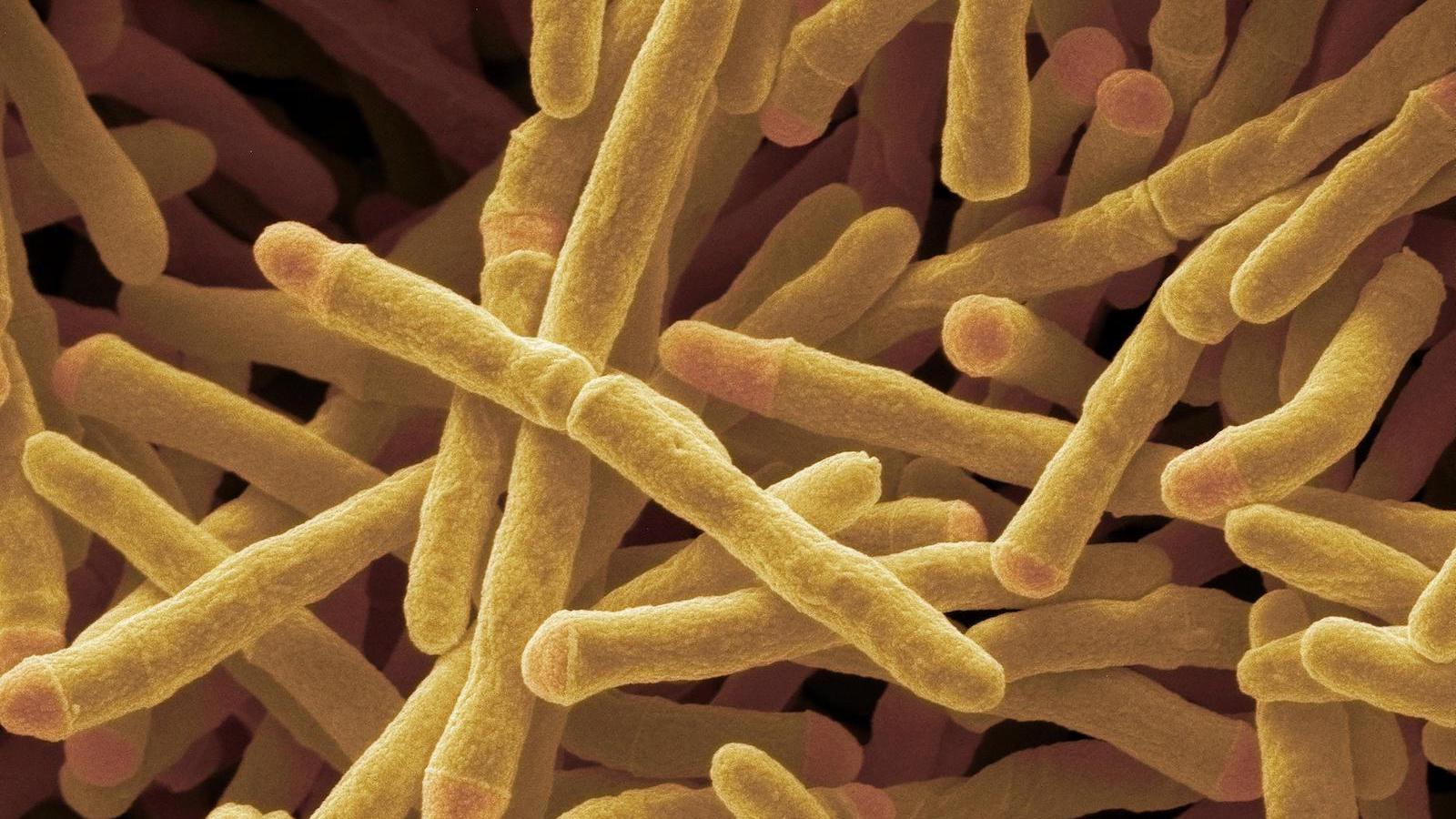
Scientists studying a cousin of the bacteria responsible for tuberculosis and leprosy have discovered an enzyme that converts hydrogen into electricity, and they think it could be used to create a new, clean source of energy literally from thin air.
The enzyme, which has been named Huc, is used by the bacterium Mycobacterium smegmatis to draw energy from atmospheric hydrogen, enabling it to survive in extreme, nutrient-poor environments.
Now, by extracting and studying the enzyme, the researchers say they have found a new energy source that could be used to power a range of small portable electrical devices. They published their findings March 8 in the journal Nature.
Related: Scientists find 'secret molecule' that allows bacteria to exhale electricity
"We imagine that a Huc-containing power source could power a range of small portable devices using air, including biometric sensors, environmental monitors, digital clocks, and calculators or simple computers," lead author Rhys Grinter, a microbiologist at Monash University in Australia, told Live Science via email.
"When you provide Huc with more concentrated hydrogen, it produces more electrical current," he said. "Which means you could use it in fuel cells to power more complex devices, like smart watches, or smartphones, more portable complex computers, and possibly even a car."
M. smegmatis is a nonpathogenic, fast-growing bacterium often used in the lab to study the cell wall structure of its close, disease-causing relative, Mycobacterium tuberculosis. Commonly found in soil all over the world, M. smegmatis has long been known to convert trace hydrogen in the air into energy; in this way, the microbe can survive in the toughest environments, including Antarctic soils, volcanic craters and the deep ocean, where little other fuel can be found, the researchers said.
Get the world’s most fascinating discoveries delivered straight to your inbox.
But until now, how M. smegmatis did this was a pervading mystery.
To investigate the chemistry behind M. smegmatis' shocking ability, the scientists first isolated the Huc enzyme responsible for the process using chromatography — a lab technique which enables scientists to separate the components of a mixture. Then, they investigated the enzyme's atomic structure with cryo-electron microscopy, a technique that won its creators the 2017 Nobel Prize in chemistry. By beaming electrons onto a frozen sample of Huc that was gathered from M. smegmatis, the researchers mapped out the enzyme's atomic structure and the electrical pathways it uses to carry the electrons so that they form a current.
The team discovered that at its center, Huc has a structure, called an active site, that contains charged ions of nickel and iron. Once hydrogen molecules (made up of two protons and two electrons) enter the active site, they become trapped between the nickel and iron ions and get stripped of their electrons. The enzyme then sends these electrons along in a flowing stream to generate a current.
"The electrons are absorbed by Huc (specifically the nickel ion), and transferred to the surface of Huc (by a molecular wire formed by clusters of iron and sulphur ions)," Grinter said. "If we immobilize Huc on an electrode, the electrons can enter an electrical circuit from the enzyme surface and generate current."
Further experiments revealed that the isolated Huc enzyme can be stored for prolonged periods; that it survives being frozen or heated up to 176 degrees Fahrenheit (80 degrees Celsius); and that it can consume hydrogen at concentrations as minuscule as 0.00005% of that found in the air we breathe. These attributes, alongside the microbe's ubiquity and ability to be easily grown, could make the enzyme an ideal candidate for a power source in organic batteries, the researchers say.
"Huc can extract energy from hydrogen in the air, which is effectively limitless," Grinter said. "The amount of electricity that can be generated from the low concentrations of hydrogen in the air will be modest. This will limit the application of Huc in this context to devices that require a small but sustained amount of power. A complementary use of Huc would be in fuel cells where a higher concentration of hydrogen is provided."

Ben Turner is a U.K. based writer and editor at Live Science. He covers physics and astronomy, tech and climate change. He graduated from University College London with a degree in particle physics before training as a journalist. When he's not writing, Ben enjoys reading literature, playing the guitar and embarrassing himself with chess.
 Live Science Plus
Live Science Plus










