Scientists zoom in on HIV inside a test tube, find critical steps in infection

Scientists have finally recreated the initial steps of HIV infection in a test tube, offering an incredibly zoomed-in view of the virus in action.
The amazing images show a cone-shaped shell of geometric tiles, called the capsid, that sits at the virus's center and contains its genetic material, known as RNA. Before infiltrating a cell, the capsid is surrounded by an envelope of fatty molecules; this envelope fuses to the host cell to let the capsid inside, where it then carries the RNA to the cell's nucleus. On the way, the RNA replicates, and once inside the nucleus, it invades the host's DNA.
By granting a closer look at this replication process, the new study highlights that the capsid itself plays a critical role in infection and that specific criteria must be met for the virus to interweave its genome with the host cell's.
Knowing how to recreate the initial steps of HIV infection "means we have many more tools for dissecting the process of replication," said study author Wesley Sundquist, a distinguished professor of biochemistry at University of Utah Health. In particular, the study, published Oct. 8 in the journal Science, describes a cell-free system that can be used to study how HIV invades the host genome — such a system could "revolutionize HIV experiments in many labs," Leo James, a group leader at the MRC Laboratory of Molecular Biology, who was not involved in the study, told Live Science in an email.
"To have accomplished all this is a real tour de force," James said. Beyond basic research, the system could also help explain how experimental drugs that target the capsid work to limit HIV replication, Sundquist said in a statement.
Related: 12 amazing images in medicine
Test tube trials
Although the test tube experiments capture aspects of HIV infection in superb detail, they cannot recreate every step in the process, Sundquist noted. Infection typically starts when the outer membrane of the virus fuses with the membrane of a host cell, allowing the capsid and its innards to sneak inside. But with a cell-free system, the authors had to bypass this initial step.
Get the world’s most fascinating discoveries delivered straight to your inbox.
They instead used a compound found in bee venom, called melittin, to "permeabilize" the viral membrane and release the capsid held within.
The HIV capsid has tiny pores in it, and normally, as a virus particle floats through a human cell's cytoplasm, it picks up the cellular building blocks of DNA, called deoxynucleotide triphosphates, that are already there, according to a 2017 report in the journal Nature. As it travels to the nucleus, the virus particle uses those building blocks to make copies of full strands of DNA, thanks to a special enzyme housed inside the capsid. This is how the virus copies its genetic material to later insert into the host genome. How the virus "knows" when to start this so-called reverse transcription is still somewhat mysterious, but studies hint that biochemical properties of the host cell act as cues for the reaction to begin.
But a test tube doesn't automatically have DNA building blocks in solution, so to jumpstart reverse transcription, the authors added them. "This method has been around for a while, but it's tricky to get the reaction to go until completion," James noted. But the study authors managed to get reverse transcription running smoothly; to do so, they learned that the capsid must stay mostly intact throughout the process.
Related: Going viral: 6 new findings about viruses
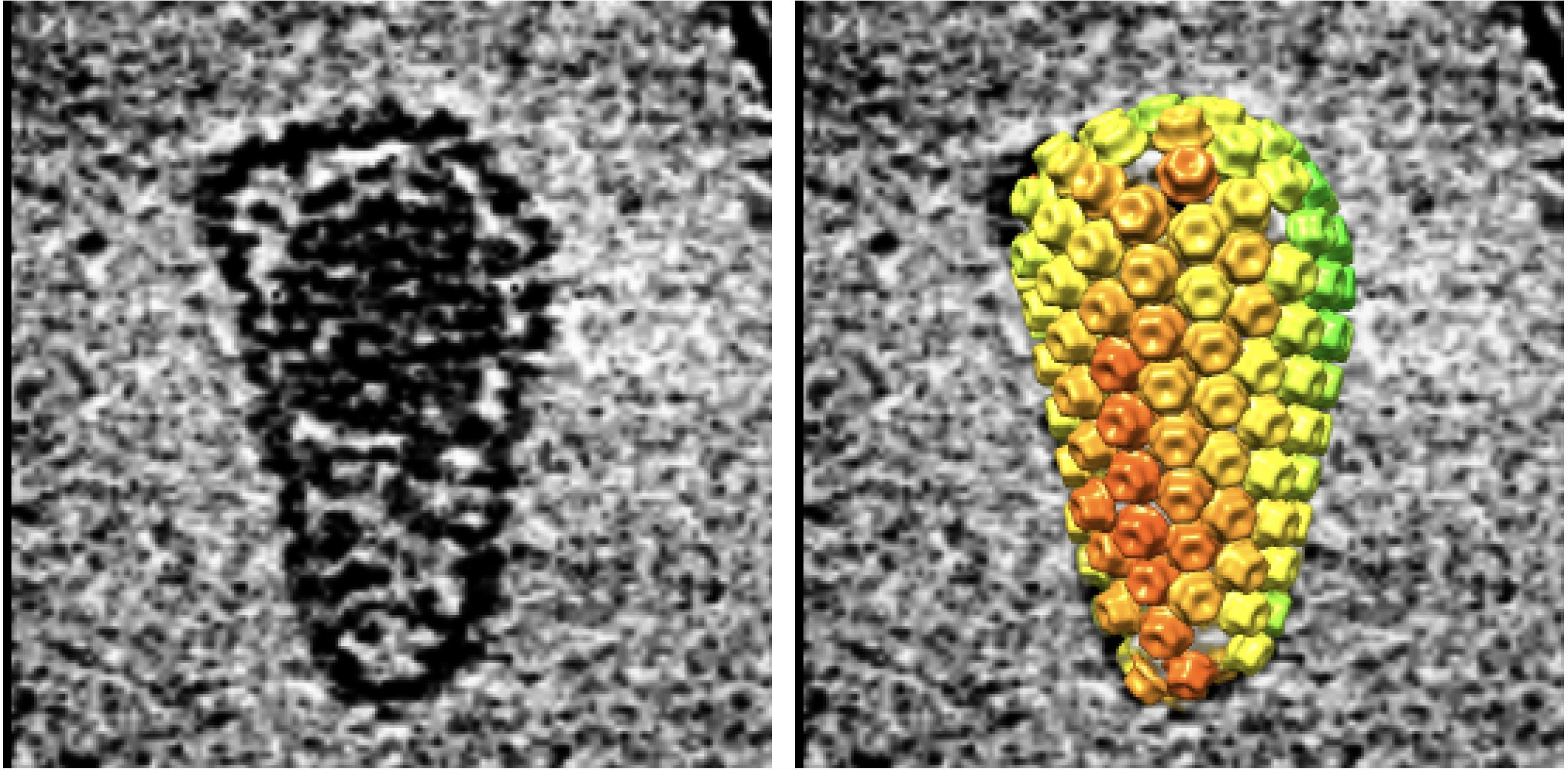
"The capsid has to be largely intact, and it has to be of the proper stability or flexibility, to support reverse transcription," meaning the capsid must be bound tightly enough to not fall apart during reverse transcription, but able to open up when the capsid enters the nucleus, in order to unleash its copied DNA, Sundquist said. Fortunately, scientists recently discovered a way to keep the capsid just stable enough.
Study author Owen Pornillos, an associate professor of molecular physiology and biological physics at the University of Virginia, and his colleagues found that a compound called IP6 binds to the capsid's tiled surface, they reported in 2018 in the journal Nature. IP6 carries a negative charge, while each tile carries a positive charge on the side that points in toward the center of the capsid; since opposites attract, when IP6 binds to the capsid, it helps pull the tiles into a tighter, more stable arrangement.
"Before the discovery of IP6, someone would remove the [viral] envelope in vitro and everything would fall apart and they could not see anything," said João Mamede, an assistant professor in the Department of Microbial Pathogens and Immunity at Rush University, who was not involved in the study.
IP6 is "quite abundant" in cells, so in their test tube experiments, the authors added similar concentrations of the compound as would be found in cells, Sundquist said. "That was really the trick," he added. "Until we knew it, we were working with capsids that were far too unstable."

Seeing is believing
Using computer models of molecules and an electron microscope, the authors could literally see that the 240 tiles making up the capsid held their lattice-like structure stable throughout reverse transcription. As the DNA strands grew larger, their ends sometimes poked through tiny gaps in the lattice weave, the authors noted, and sometimes singular tiles could be seen dislodging while the rest of the capsid stayed intact.
The capsid may need to stay stable to keep the RNA and transcribing enzyme close to each other, said Christopher Aiken, a professor of pathology, microbiology and immunology at Vanderbilt University, who was not involved in the study. The enzyme tends to fall off the RNA during transcription, so "by keeping the enzyme contained, it can rebind the template and continue DNA synthesis," Aiken told Live Science in an email.
With reverse transcription complete, the authors then moved on to the next step in infection: integration, where the viral DNA infiltrates the host genome. They introduced DNA strands known as plasmids into their test tubes, to serve as proxies for the DNA in a human nucleus, but integration would not begin without an additional ingredient. Only "whole cell extracts," a mix of proteins and molecules drawn from cells, would allow the viral DNA to pervade the plasmids.
In the future, the team hopes to pinpoint precisely which ingredients in the cell extracts trigger integration, Sundquist said. "It's likely to be more than one thing," he noted. One challenge is that, in test tube experiments, "it's always difficult to know if you're missing something," he said.
Related: The 12 deadliest viruses on Earth
One limitation of the study is that it can't perfectly recreate cellular conditions, James said.
"Any in vitro system, however powerful, can only be used to test the components we know about and can add into the reaction," James said. For instance, in actual cells, the capsid must travel to the cell nucleus, where the DNA is held, and then slip through portals known as the nuclear pores. There may be unknown factors that alter the capsid during this journey, Sundquist noted.
That said, the new cell-free system could help reveal the identity of those unknown factors, Mamede added. Scientists can now make observations in a cell-free environment and then check to see if the same behavior appears in actual cells, he said.
In addition, the system could be useful in drug development. "You can test [new drugs] more readily with one of these simplified systems than with a cell," Mamede told Live Science. "This way, you can see mechanistically what it's really doing to the virus."
The pharmaceutical company Gilead Sciences currently has a new drug in human trials that targets the HIV capsid specifically, according to ClinicalTrials.gov. Based on early data, the drug appears to alter the capsid at various points of infection, including during reverse transcription. Sundquist said that the cell-free study underscores that the capsid is a "critical component" of HIV infection, and that corrupting the capsid can limit the virus's ability to multiply.
Originally published on Live Science.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus