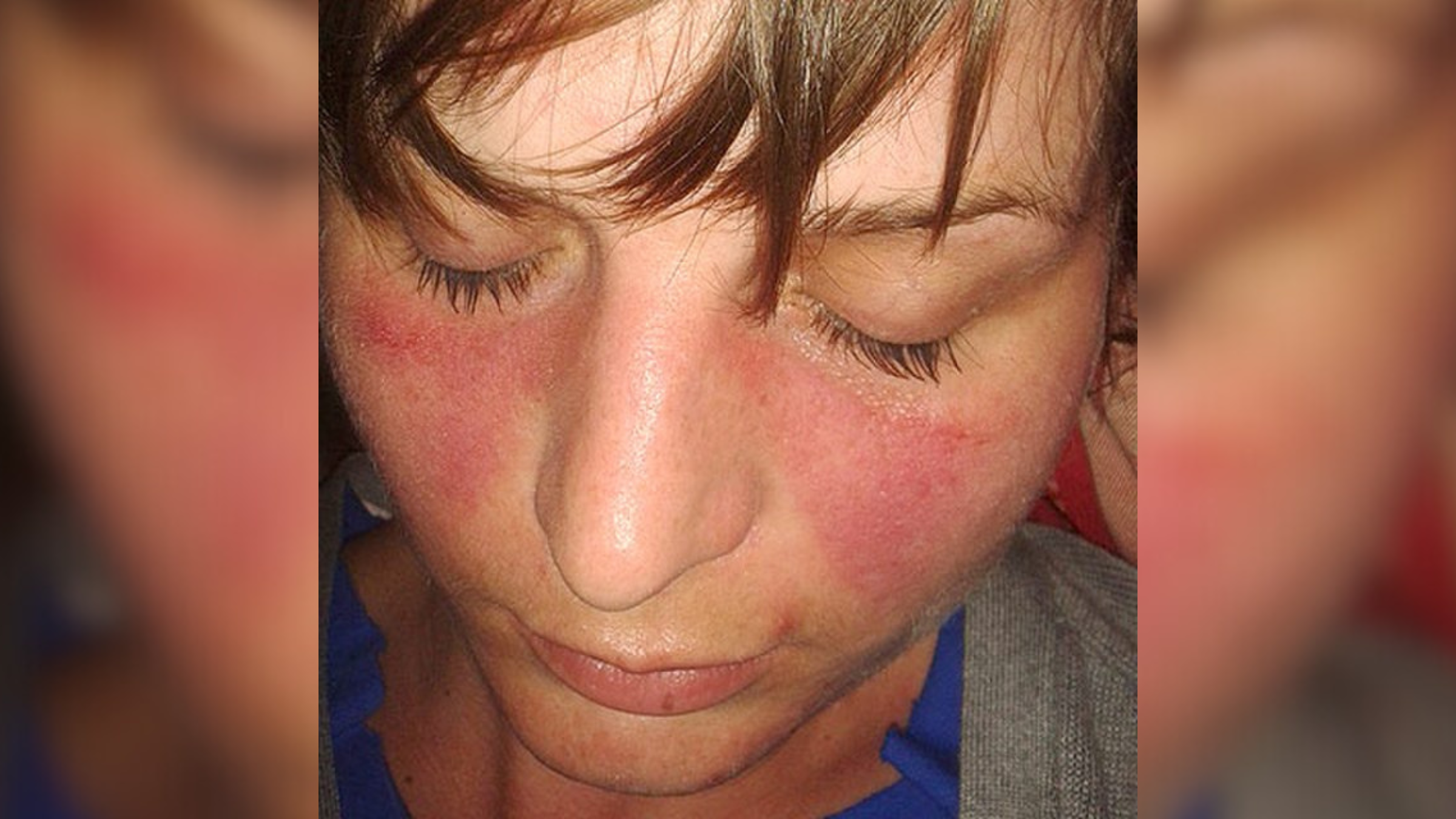
Women have 4 times men's rate of autoimmune disease. The X chromosome may be to blame.
A "complex" that regulates gene activity in people with two X chromosomes may predispose them to autoimmune disease, a new study hints.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Women are up to four times more likely than men to be affected by autoimmune disease, conditions in which the immune system mistakenly attacks the body's own cells. Now, scientists think they know why: Women's outsized risk may be tied to how the body controls its X chromosomes.
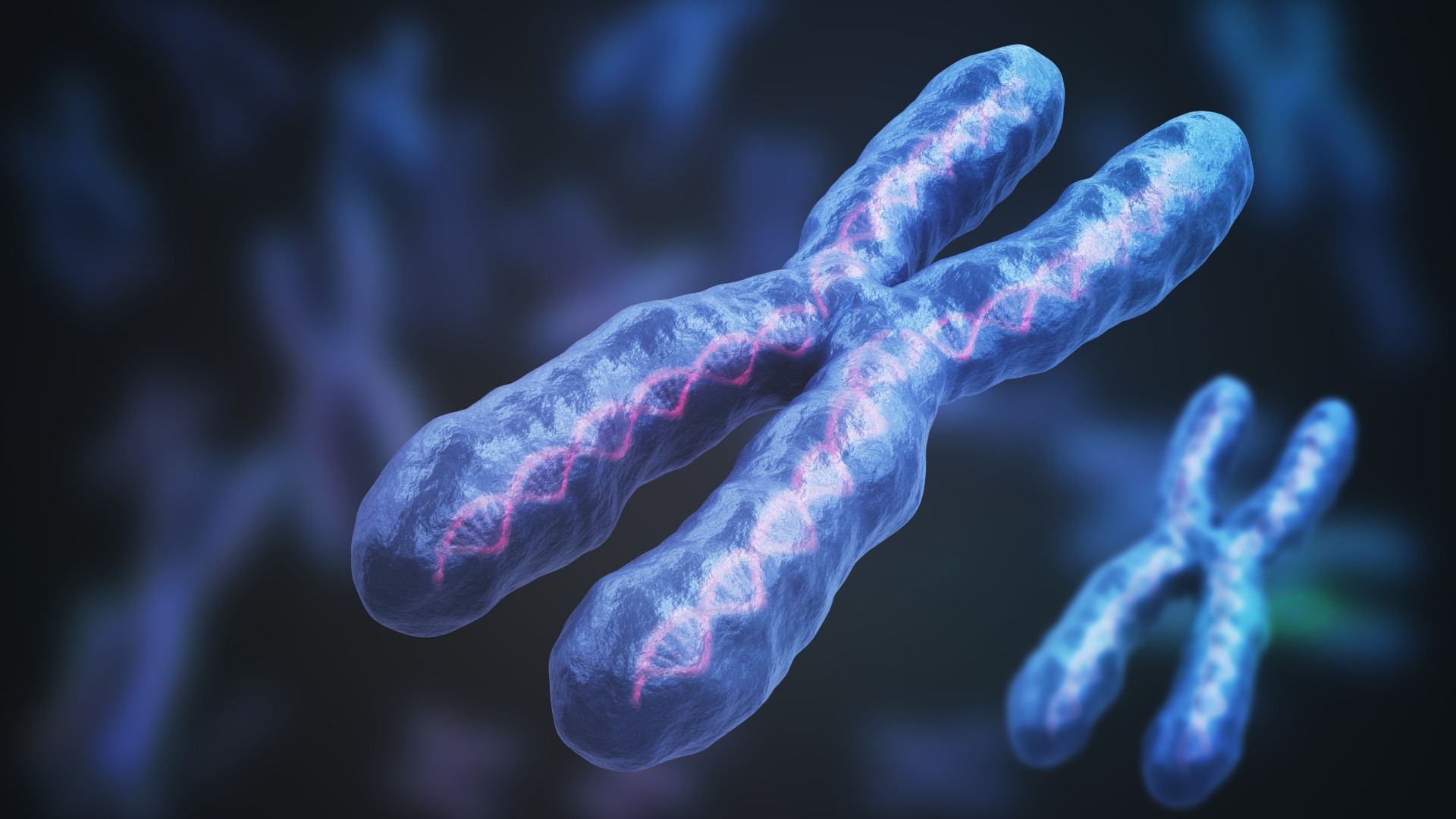
Humans have two types of sex chromosomes: X and Y. Most females carry two X chromosomes in each cell, while most males have an X and a Y. The X chromosome is larger than the Y and contains far more genes that code for proteins. But in people with two X chromosomes, only one needs to participate in protein production — otherwise, cells could soon be overwhelmed with too many proteins. To prevent this, one X chromosome in each cell is "silenced" in females during embryonic development.
A long molecule of RNA — the genetic cousin of DNA — called Xist executes this silencing by latching onto one X chromosome. It turns out, however, that many proteins are prone to sticking to Xist, and these big complexes of RNA and proteins may predispose females to autoimmune disease.
That's because the complexes can set off an immune reaction in which the body makes antibodies against the proteins within it, according to a new study in mice and humans published Thursday (Feb. 1) in the journal Cell.
"So besides it's [Xist] job in controlling gene activity, there's really a major immunological imprint that maybe hadn't previously been recognized," Dr. Howard Chang, co-senior study author and a professor of cancer research and genetics at Stanford University, told Live Science.
These findings could therefore open up new avenues for research into treatments for autoimmune diseases, he said.
Get the world’s most fascinating discoveries delivered straight to your inbox.
Autoimmune diseases, which affect more than 23.5 million Americans, are caused by a combination of genetic and environmental triggers. Scientists have proposed many theories to explain why women are more likely to develop the conditions, pointing to their hormones and the microbes inside and on them, but none of these ideas have been conclusively confirmed.
Earlier research by Chang and colleagues suggested that the Xist complex may drive sex-biased autoimmunity, as many proteins associated with autoimmune diseases could bind to it. But Xist needed to be studied in isolation, without other factors, such as hormones, that could potentially mask its influence.
So the team genetically engineered two strains of male mice to make Xist: one that was genetically susceptible to autoimmune symptoms similar to those of lupus and another that was resistant — the comparison group. In the lupus-prone strain, female mice were more prone to symptoms than male mice were, so the team theorized that Xist would bring the males' levels of disease up to that of females.
In their experiments, the team sewed a special version of the Xist gene into the genomes of male mice that could be switched on but wouldn't silence their only X chromosome. To stimulate autoimmune disease, they had to expose the lupus-prone mice to a specific chemical.
Once Xist was activated and lupus was induced, the team saw that male mice that expressed Xist developed disease at a similar rate to females and had more severe disease than mice without Xist.
However, requiring both the environmental chemical trigger and a genetic predisposition to lupus was an important control, Chang said. That made the mouse experiments more relevant to humans.
"If someone is born with a genetic susceptibility, then the presence of Xist has some impact but also, very importantly, this environmental trigger [is necessary]," Chang said. Carrying Xist doesn't guarantee a person will have an autoimmune condition; the Xist complex may just account for the discrepancy in case counts between the sexes.
To back up their mouse results, the team analyzed blood samples from more than 100 patients with autoimmune diseases, including lupus, and 20 without autoimmune disease. They discovered that the patients with autoimmunity had more Xist autoantibodies in their blood than the individuals without autoimmunity did.
The types and numbers of autoantibodies in different people were disease-specific, which may help with the future diagnosis and treatment of these conditions, Chang said. For example, someday, taking these autoantibody profiles could help doctors decipher which disease a patient has or predict the trajectory of their condition, he said.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.
 Live Science Plus
Live Science Plus