At-home flu vaccine approved by FDA — what to know
People could previously get the nasal spray flu vaccine, called FluMist, from a health care provider, but now they can administer it themselves.
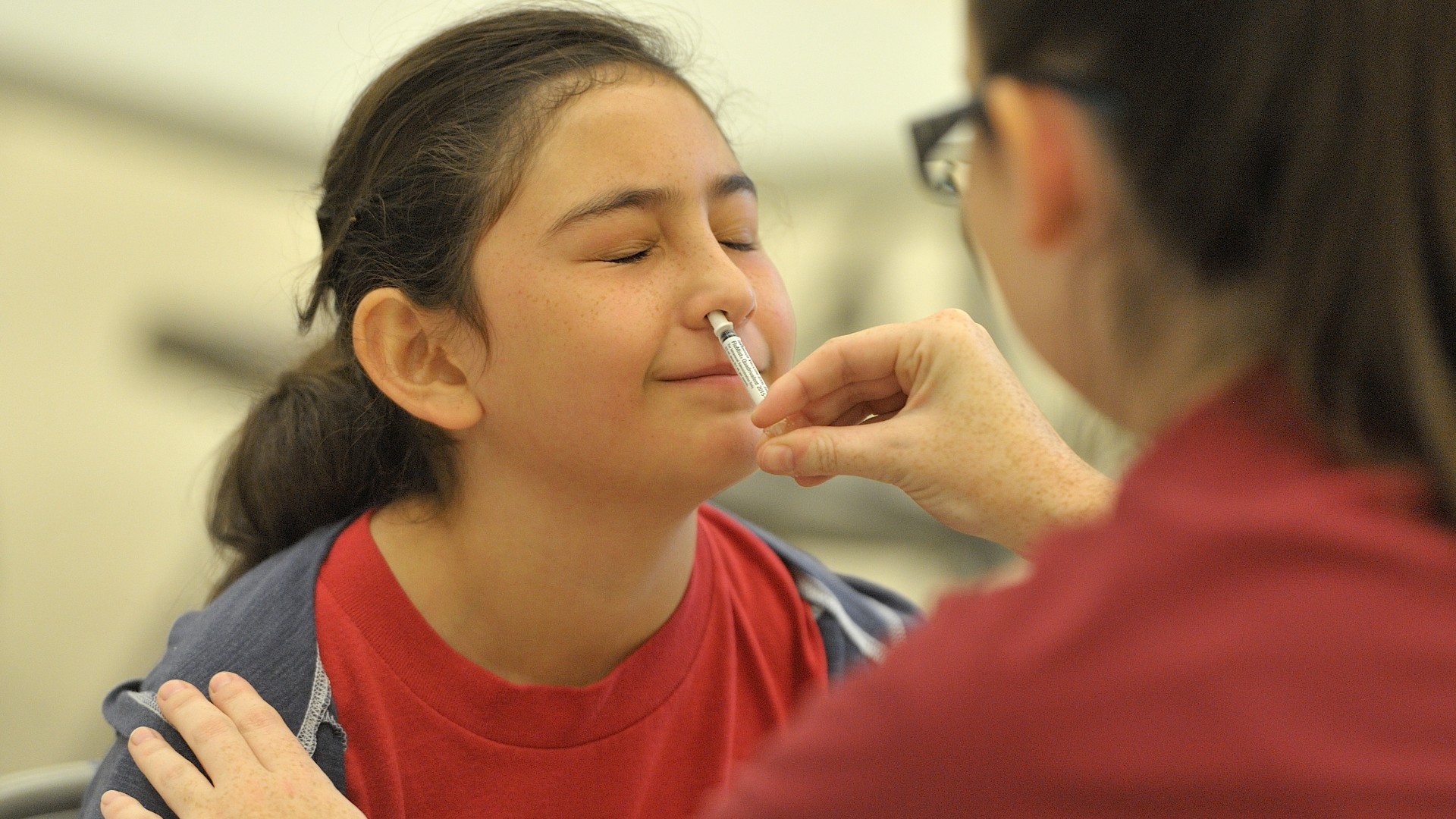
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
A nasal spray version of the annual flu vaccine can now be taken at home, the U.S. Food and Drug Administration (FDA) says.
In a statement published Sept. 20, the FDA announced that it had approved the nasal spray influenza vaccine, called FluMist, for use at home. This allows people ages 18 to 49 to self-administer the vaccine — meaning they spray it up their own nose. The vaccine is also cleared for children and adolescents ages 2 to 17, whose caregivers can administer the spray.
FluMist was previously available for people ages 2 to 49 in the U.S., but it could be administered only by professionals in health care settings, such as pharmacies and doctors' offices. The FDA's new announcement comes after clinical trial data showed that FluMist was easy to self-administer and that it was just as safe and effective to vaccinate this way as it was by health care professionals.
The U.S. Centers for Disease Control and Prevention (CDC) recommends that, except in rare circumstances, everyone 6 months and older get a flu vaccine each season — ideally between September and October, before the flu starts circulating widely. The new approval could make vaccination more convenient, flexible and accessible, Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in the statement.
Related: 2-in-1 shot for flu and COVID shows promise in advanced trial
"Getting vaccinated each year is the best way to prevent influenza, which causes illness in a substantial proportion of the U.S. population every year and may result in serious complications, including hospitalization and death," Marks said. "This approval adds another option for vaccination against influenza disease and demonstrates the FDA's commitment to advancing public health."
Here's what we know so far about using FluMist at home.
Get the world’s most fascinating discoveries delivered straight to your inbox.
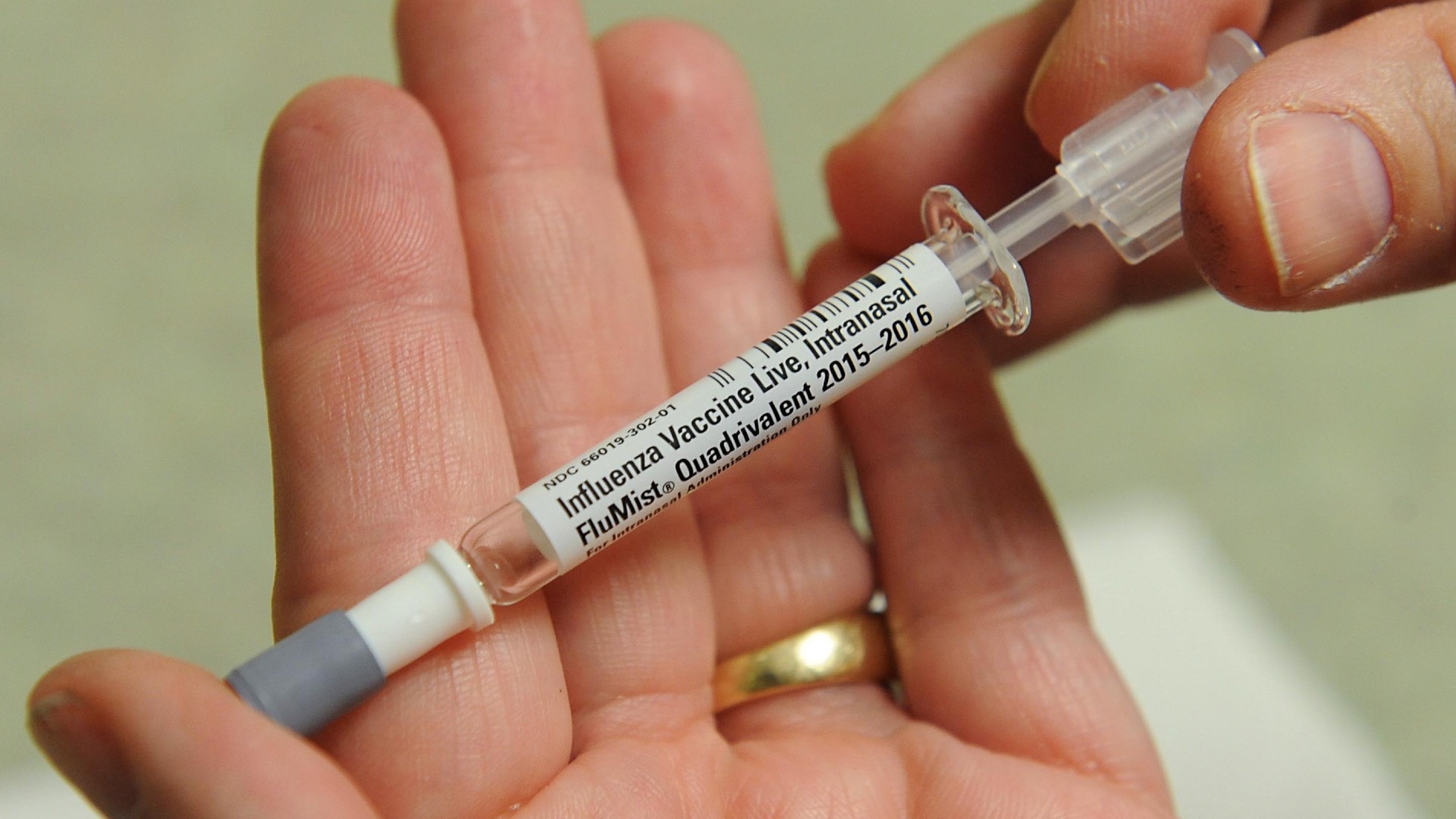
What is FluMist?
FluMist is a nasal spray-based vaccine that helps prevent illness caused by two types of flu, known as influenza A and B. These virus types are responsible for most flu infections in humans.
FluMist is a live, attenuated vaccine, meaning it contains weakened versions of influenza A and B viruses. These weakened viruses cannot cause the flu; instead, they prime the immune system to produce antibodies to fight off future infections.
FluMist first gained FDA approval in 2003, for use in people ages 5 to 49. In 2007, this approval was expanded to include children as young as 2. Until now, FluMist could be administered only by health care providers in pharmacies and other health care facilities.
FluMist is the only non-injected flu vaccine available to people in the U.S. For the 2024-2025 flu season, FluMist and all of the other approved vaccines will guard against three subtypes of flu: two influenza A viruses and one influenza B virus.
How do you use FluMist?
FluMist is administered via one spray in each nostril. The vaccine is packaged in a small tube with a plunger on one end. The end of the tube opposite the plunger goes into the nose, and then you push the plunger to release the spray, emptying one half of the tube's contents into each nostril.
People ages 9 and older require only one dose of the vaccine per year — meaning one spritz in each nostril. However, children ages 2 to 8 may need an additional dose at least a month later. This depends on the child's vaccination history; children who haven't gotten at least two doses previously or whose vaccination history is unknown need two doses.
In these instances, your health care provider can help determine whether your child needs a second dose.
How do I order FluMist?
Although the FDA has approved FluMist for use at home, it will take some time for the spray to be available for home delivery.
The vaccine's maker, AstraZeneca, said it anticipates the vaccine will be available to order for home delivery through a third-party online pharmacy service in time for the 2025-2026 flu season. The FDA notes you still need a prescription for FluMist — in this case, that means a pre-approved prescription provided by an online pharmacist.
Is there anyone who shouldn't get FluMist?
There are several groups of people who should not get a FluMist vaccine, according to the CDC. They include individuals who are pregnant and those with weakened immune systems. These precautions are taken because the FluMist vaccine contains live (albeit weakened) viruses, while flu shot options do not.
Although not everyone can use FluMist, it is hoped that the new approval will make needle-free options for flu vaccines available to more people.
"It's very exciting to see the FDA take this step and find one more way to make it easy for people to get vaccines," said Dr. Andrew Handel, a pediatric infectious diseases expert at Stony Brook Children's Hospital in New York.
It's possible that the new approval may even inspire pharmaceutical companies to pursue the development of nasal vaccines for other respiratory infections, such as COVID-19, he told Live Science.
This article is for informational purposes only and is not meant to offer medical advice.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line "Health Desk Q," and you may see your question answered on the website!

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.
 Live Science Plus
Live Science Plus










