Should we rethink our legal definition of a human embryo?
Scientists can now create realistic human embryo models in the lab, leading some to suggest that we rethink how we legally define an embryo.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
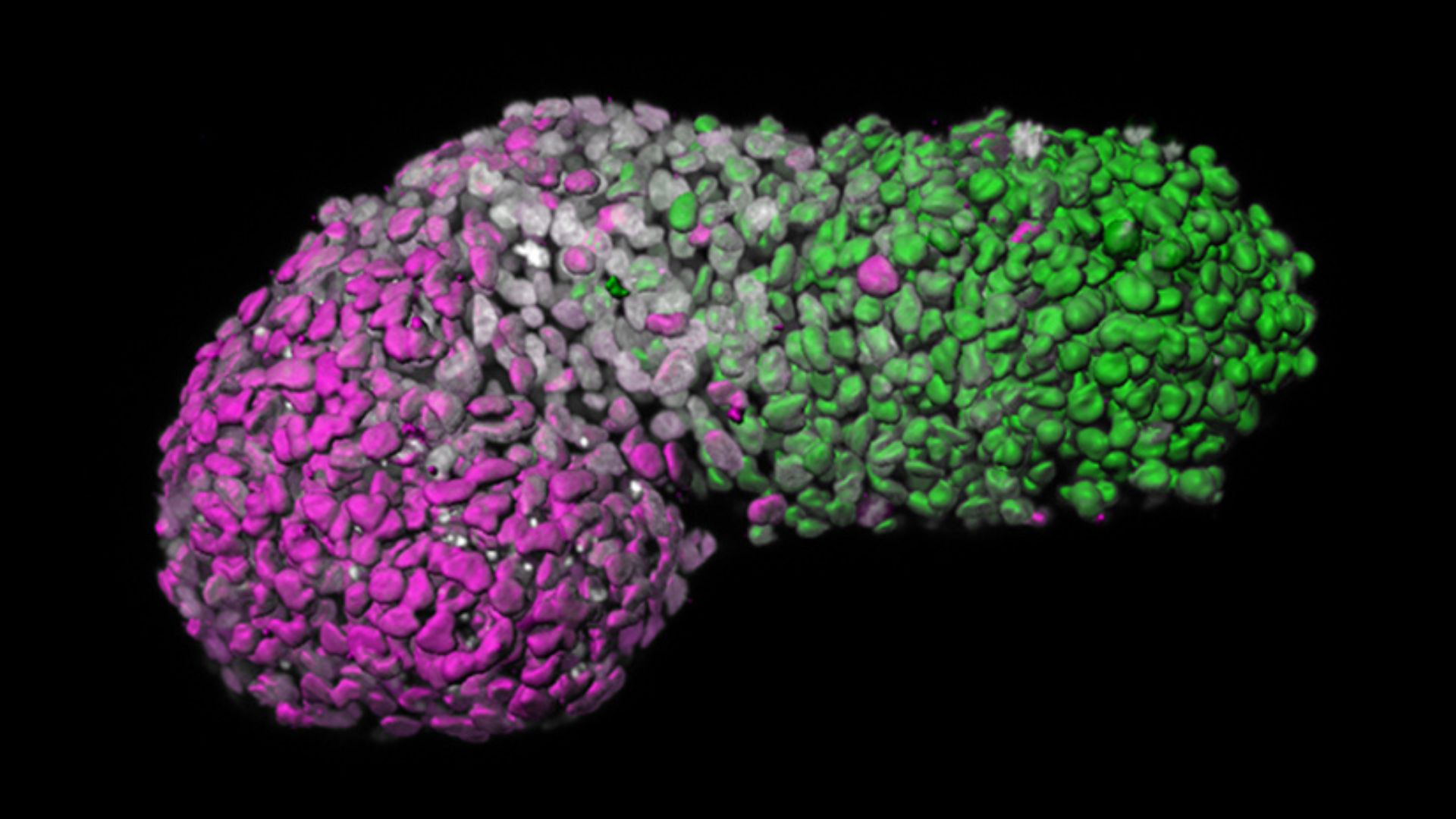
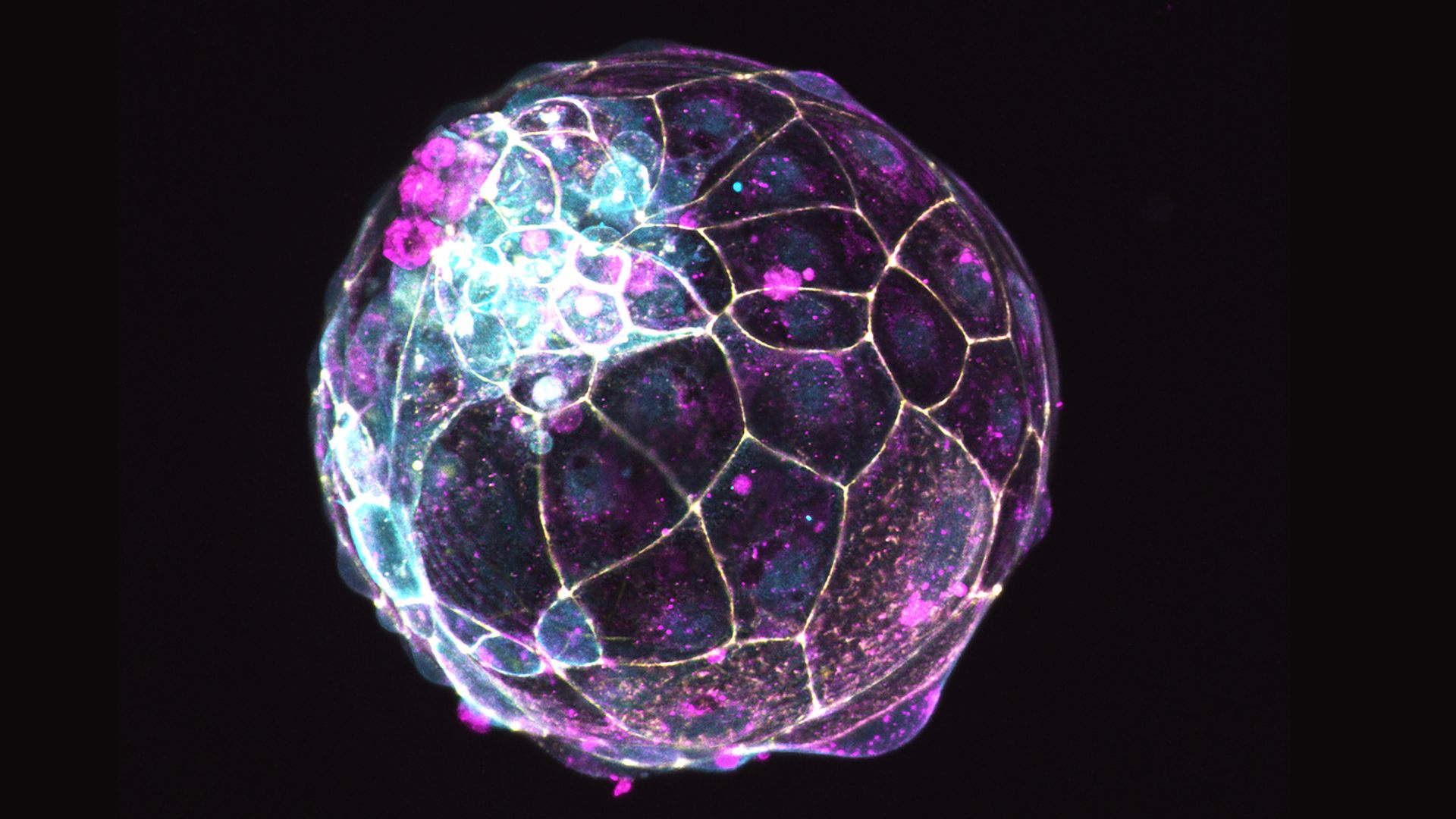
Scientists can now create embryo-like structures from animal and human stem cells in the lab, and recently, researchers unveiled the most advanced human embryo models yet, which resemble natural embryos up to 14 days after fertilization.
But this research has raised legal and ethical questions about what an embryo is and how lab-made models should be used. So some scientists argue that the legal definition of an embryo should be changed.
In the U.S., a 1996 amendment that bars federal funding for human embryo experiments defines a human embryo as an organism derived by fertilization, cloning, parthenogenesis — when an unfertilized egg develops into an embryo — or by any other means from one or more human sex cells or "diploid" cells, which contain a full set of chromosomes. That definition excludes current stem-cell-derived "embryo models."
But in a paper published Aug. 17 in the journal Cell, researchers argue embryos should be defined based on whether they can become a living organism, rather than how they were made. They propose that a human embryo be defined as "a group of human cells supported by elements fulfilling extraembryonic and uterine functions that, combined, have the potential to form a fetus." ("Extraembryonic" refers to tissues like the placenta and yolk sac.)
Embryo models that could develop into fetuses would fall into this category and be afforded similar protection as true embryos. The paper authors also want to pin down potential moments, or "tipping points," when an embryo model would legally become a fully-fledged embryo.
Figuring out this legal definition is important because the technology for carrying these self-organizing structures further into development, up to the stage where an embryo becomes a fetus and beyond, could be here within a decade, the scientists say. And without those legal tipping points defined, some scientists would be free to potentially carry these models far into gestation, or even attempt to create a living human from them.
Get the world’s most fascinating discoveries delivered straight to your inbox.
What are embryo models and why are they being made?
Embryo models are typically made from genetically tweaked or chemically treated stem cells, which are either embryonic or derived from adult human cells. Under carefully controlled conditions, scientists can "persuade" these stem cells to self-organize into blobs of tissue that resemble different stages of early embryonic development, said Roger Sturmey, a professor in reproductive medicine at Hull York Medical School in the U.K. who was not involved in the Cell paper.
Models called blastoids can recreate one of the earliest stages of embryo development — a hollow ball of cells called a blastocyst — at around five days after fertilization. More-complex models, called gastruloids, model gastrulation, when the embryo goes from being made of just one cell layer to becoming a 3D structure around two weeks after fertilization.
Theoretically, gastruloids could be grown in a lab for more than 14 days. But they generally aren't, due to a "14-day rule" upheld by law or by research regulations in various countries. Some researchers, including an influential scientific panel, have called for the rule to be loosened to allow for embryos and embryo models to be grown for longer.
These models could provide a detailed look at the first 30 days of embryonic development, enabling scientists to improve the efficiency of fertility treatments such as in vitro fertilization (IVF) and uncover causes of early miscarriage, Nicolas Rivron, lead author of the Cell paper and a principal investigator at the Institute of Molecular Biotechnology in Vienna, told Live Science. They could also reveal how development in the womb influences health in adulthood — for example, how it might affect the risk of developing cardiovascular diseases or diabetes, he added.
Such research would be technically challenging and unethical to do in a pregnant person, making embryo models especially appealing to researchers studying early development.
Why change the definition now?
Early definitions of embryos always included eggs and sperm. These ingredients would give rise to a group of cells that self-organized and could form an offspring.
But as embryo-like structures become more similar to real embryos, scientists are realizing that within possibly a decade, such models could, in theory, contain all the elements to give rise to living organisms with no eggs or sperm required, Rivron said.
"The question that comes now is whether we should adjust the definition based on potential advances in the next decade or decades," Rivron told Live Science.
As we don't have a clear understanding of when an embryo model would actually become an embryo, Rivron said, he and his colleagues want to define "tipping points" when that line could be considered crossed. One test would assess whether a fetus could feasibly be grown from an embryo model in the lab. Another would see whether similar animal embryo models could develop into live animals when implanted into hosts. These tests would, of course, be ethically regulated by authorities, the authors said.
Related: Part-human, part-monkey embryos grown in lab dishes
How might a change affect medical research?
"These embryo models are a great scientific and ethical opportunity," Rivron said. That's because they're easier to produce and study than embryos embedded in a womb.
"But we have to make sure that all of this is done within a proper framework that fits with societal values," Rivron stressed. "We think that as long as an embryo is capable of forming a living organism, then it should definitely be considered an embryo, regardless of how it was made," he said.
In their paper, the authors argue that the development of human embryo models in the lab should happen gradually and only be allowed to progress to later stages of development if initial quality checks are passed. They hope that this will help limit unethical research and provide space to reflect on the potential benefits that these models could bring to society.
"As we further our understanding, like in any field that challenges what's gone before, we have to reflect on what we've known on the basis of the information that we currently have available," Sturmey said. "It's time to have that dialogue."

Emily is a health news writer based in London, United Kingdom. She holds a bachelor's degree in biology from Durham University and a master's degree in clinical and therapeutic neuroscience from Oxford University. She has worked in science communication, medical writing and as a local news reporter while undertaking NCTJ journalism training with News Associates. In 2018, she was named one of MHP Communications' 30 journalists to watch under 30.
 Live Science Plus
Live Science Plus