Most advanced lab-made human embryo models look like the real thing
New models of human embryos grown in the lab closely mimic the structure of actual embryos.
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
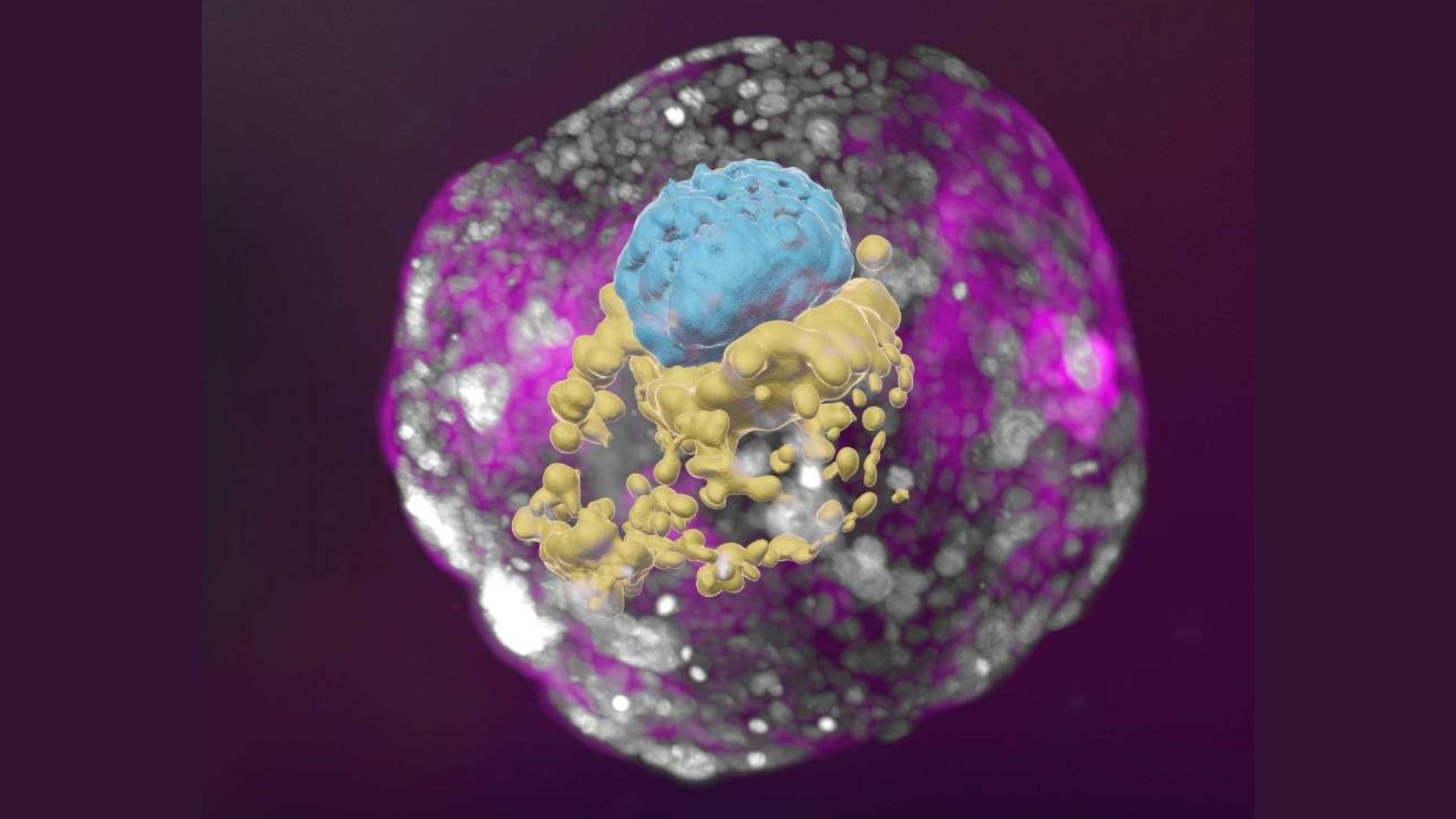
The most advanced lab-made human embryo models look like the real thing — they resemble, though don't perfectly replicate, natural embryos about 14 days into development.
These lab-made embryo models open a window into the earliest stages of human development, when a fertilized egg first starts dividing and implants in the wall of the uterus. Researchers hope such models will be useful for studying birth defects that emerge early in development, reasons pregnancies can fail at this stage, and how drug exposures affect developing embryos.
The team behind these embryo-like spheres of cells is led by Jacob Hanna, a stem cell biologist at the Weizmann Institute of Science in Israel. The researchers first announced that they'd grown 14-day embryo models back in June on the preprint database bioRxiv, amid a flurry of other yet-unreviewed papers about embryo models made by three other groups. Now, the paper by Hanna and his team has been published in the peer-reviewed journal Nature.
"In contrast to similar studies published earlier this year, these embryo-like structures contained most of the cell types found in developing embryos," Darius Widera, a professor of stem cell biology and regenerative medicine at the University of Reading in the U.K. who was not involved in the work, told CBS.
Related: Having a baby: Stages of pregnancy by trimester
Previously, simpler human embryo models had been grown for shorter lengths of time, and more-advanced mouse embryo models had been grown to the point where they'd started to grow brains and beating hearts. Then in June, the four research groups posted preprints — research papers yet to undergo peer review — describing human embryo models they'd cultivated to be much more advanced.
All these models start as stem cells, unspecialized cells that can give rise to a variety of cell types by taking on new traits as they divide. Some of the groups genetically tweak these stem cells to nudge them toward making an embryo and its accessory tissues, like the placenta.
Get the world’s most fascinating discoveries delivered straight to your inbox.
But Hanna's group uses only chemicals to coax stem cells to form these tissues. This approach results in a more accurate embryo model, they say, with a more realistic overall structure and different cell types, according to a statement from the Weizmann Institute.
To make their models, Hanna's team first pushes stem cells into a "naive" state, from which they can produce any cell type. These naive cells are then made to form cells of the embryo, placenta, yolk sac and "extraembryonic mesoderm membrane" — the precursor to the chorionic sac, the outermost membrane that surrounds the fetus. All of these cells get mixed together, and about 1% ultimately clump up to form balls with the distinct 3D architecture of a real human embryo.
"The similarity to the natural embryo is remarkable, almost uncanny," Jesse Veenvliet, a developmental biologist at the Max Planck Institute of Molecular Cell Biology and Genetics in Germany, told Science when the Hanna lab preprint dropped in June. By contrast, embryo models made with genetically modified stem cells have been criticized as having a very different structure than human embryos, Nature reported.
"This is the first embryo model that has structural compartment organisation and morphological similarity to a human embryo at day 14," Hanna told The Guardian.
Although the new models should be useful in research, their creation does come with ethical questions — for starters, how long should lab-made embryos be allowed to mature? Historically, scientists have generally followed the "14-day rule" that says such embryos should not be allowed to mature for more than two weeks, but some have argued that the time window should be widened. Researchers around the world are still wrestling with these questions even as the embryo models steadily become more sophisticated.

Nicoletta Lanese is the health channel editor at Live Science and was previously a news editor and staff writer at the site. She holds a graduate certificate in science communication from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, among other outlets. Based in NYC, she also remains heavily involved in dance and performs in local choreographers' work.
 Live Science Plus
Live Science Plus











