Are free radicals really that bad for you?
Free radicals are highly reactive molecules that are missing an electron. They get a bad rap, but could they actually have any benefits?
Get the world’s most fascinating discoveries delivered straight to your inbox.
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered Daily
Daily Newsletter
Sign up for the latest discoveries, groundbreaking research and fascinating breakthroughs that impact you and the wider world direct to your inbox.

Once a week
Life's Little Mysteries
Feed your curiosity with an exclusive mystery every week, solved with science and delivered direct to your inbox before it's seen anywhere else.

Once a week
How It Works
Sign up to our free science & technology newsletter for your weekly fix of fascinating articles, quick quizzes, amazing images, and more

Delivered daily
Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!
Join the club
Get full access to premium articles, exclusive features and a growing list of member rewards.
Free radicals have a terrible reputation. These reactive and unstable molecules have been associated with cancer, aging, and degenerative diseases such as Alzheimer's.
Yet the body constantly produces free radicals as a key part of life-sustaining processes, such as respiration, cell signaling and defense against disease. So are free radicals really all that bad, or are there any unappreciated benefits?
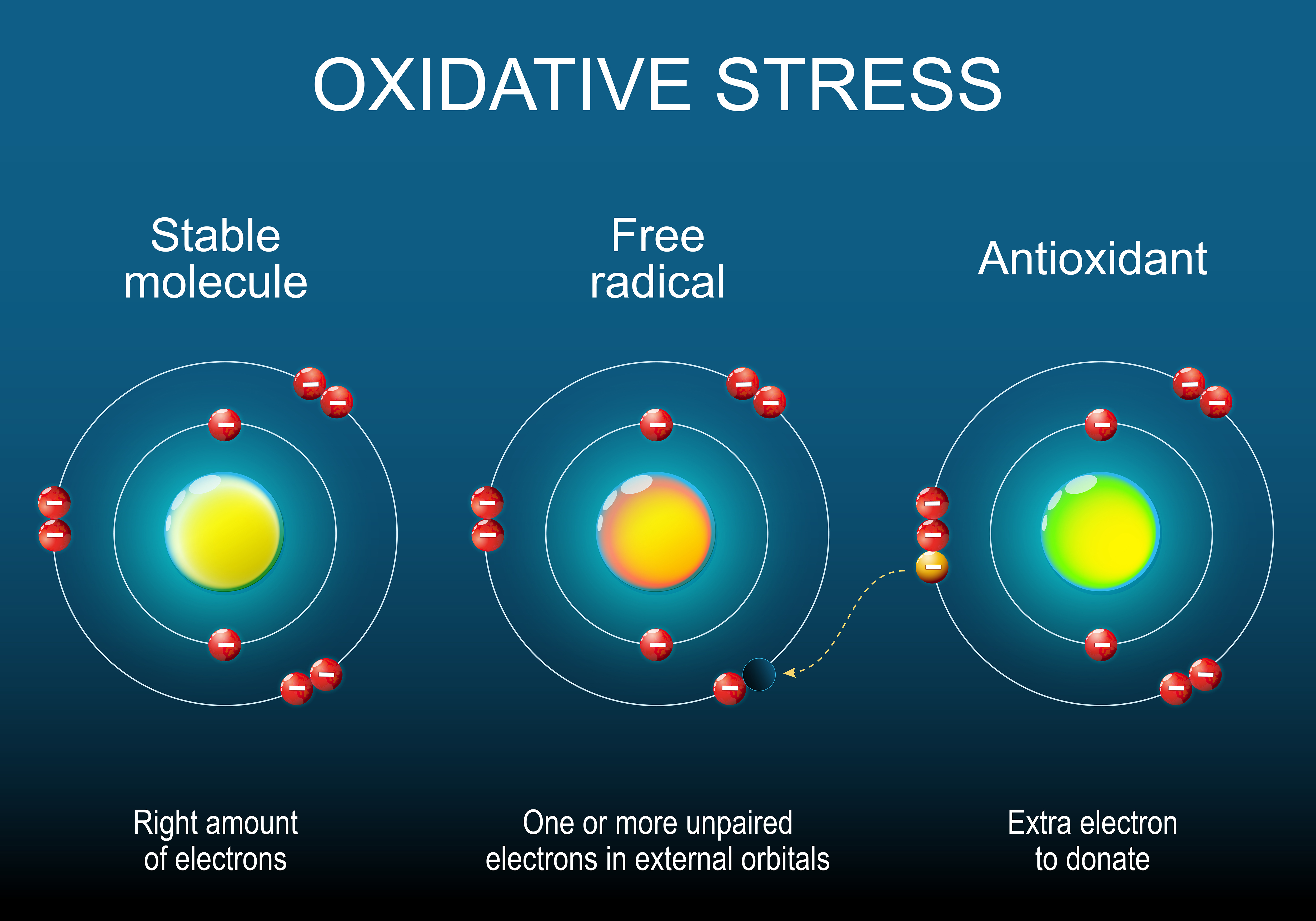
At high levels, free radicals undeniably pose a danger. Free radicals, often called reactive oxygen species (ROS), contain a single unpaired electron that must find another electron with which to form a stable electron pair. As a result, these molecules are indiscriminately reactive and will tear an electron from cell membranes, proteins, DNA or any other nearby structures, thus deforming their shape to either damage or deactivate them.
And this isn't just a one-time event, said Michael Murphy, a mitochondrial biologist at the University of Cambridge. "If a radical rips away an electron, it leaves an unpaired electron behind, and that will react on further. So you often end up with a nasty chain reaction."
However, this problematic potency can also be harnessed by the body under controlled circumstances. The immune system deploys radicals to attack pathogens and defend against disease, while other single-electron species, such as nitric oxide (NO), are used as signaling molecules for communication between different cells.
"Some enzymes use free radical chemistry inside their active sites because that gives them the ability to do chemistry that's more difficult," Murphy told Live Science. Consequently, many of the body's fundamental reactions, from respiration to oxygen transport, rely on radical intermediates to make their chemistry possible.

Sign up for our weekly Life's Little Mysteries newsletter to get the latest mysteries before they appear online.
In fact, most radicals in the body — around 90% — are generated by mitochondria, the cellular machinery responsible for respiration, said Michael Ristow, a longevity researcher at Charité University Medicine Berlin.
Respiration is the life-sustaining process that releases energy by breaking down glucose and oxygen. The overall process involves a complex sequence of steps, mediated by a series of proteins called the electron transport chain. These proteins pass electrons between different units during this reaction sequence, but a small amount of leakage means some radicals are also released into the surrounding cellular environment.
"What can happen is electrons come off these enzymes and react with oxygen to form an oxygen free radical called superoxide — that's often the proximal free radical produced in biology," Murphy explained. "It goes on to form hydrogen peroxide (which isn't a free radical), and that can be particularly damaging in the presence of the ferrous iron (Fe2+). That produces another radical called the hydroxyl radical, which will just react with anything."
All of this is part of the ordinary workings of every cell, so free radicals really become a problem only when generated in high levels, Ristow said.
"The body has evolved a number of different defense mechanisms," he explained. A healthy diet naturally includes antioxidant molecules such as vitamins C and E, which can quench these reactive particles. "Then there are dedicated enzymes that convert free radicals into less-dangerous intermediates, and there is the glutathione system," a sulfur-containing molecule that acts like a sacrificial sponge, mopping up free radicals before being recycled by another enzyme.
However, certain environmental factors such as UV exposure or excessive alcohol intake can trigger the production of higher levels of free radicals via a slightly different mechanism. "The UV can react with what are called photosensitizers. The molecule goes into an excited state as it absorbs the energy, then it will transfer that energy often to oxygen," explained Murphy. "The chemistry is a bit complicated, but you convert oxygen to a more reactive form of oxygen that then acts on fat and other double bonds."
Beyond a certain point (which is different for everyone), these extra radicals will overload the body's natural defensive mechanisms. The result: damaged tissue which over time leads to diseases such as cancer.
But crucially, there's growing evidence that controlled levels of free radicals are actually beneficial, or even essential, to health — part of a phenomenon known as hormesis.
"The response to exposure to free radicals on a systemic level is typically increased response capacity against free radicals," Ristow said. "So the entire human body is then better prepared, not only against free radicals, but also toxic food components, UV exposure and other sources of damage."
This effect is most clearly demonstrated in the context of exercise.
"If you take antioxidants before or together with exercise, the effect of exercise on health parameters is gone or massively reduced," Ristow said. Endurance, recovery, muscle gain and insulin resistance are affected, and although there are several theories around the role radicals play in each case, there's currently no consensus in the field.
Free radicals clearly have the potential to cause harm, but whether they are good or bad has a much more nuanced answer that depends on both the context and the concentration. "It's a balance," Ristow said. "But if ROS really were only damaging, then evolution would have ruled them out!"
This article is for informational purposes only and is not meant to offer medical or dietary advice.

Victoria Atkinson is a freelance science journalist, specializing in chemistry and its interface with the natural and human-made worlds. Currently based in York (UK), she formerly worked as a science content developer at the University of Oxford, and later as a member of the Chemistry World editorial team. Since becoming a freelancer, Victoria has expanded her focus to explore topics from across the sciences and has also worked with Chemistry Review, Neon Squid Publishing and the Open University, amongst others. She has a DPhil in organic chemistry from the University of Oxford.
You must confirm your public display name before commenting
Please logout and then login again, you will then be prompted to enter your display name.
 Live Science Plus
Live Science Plus











