3D-Printed Ovaries Offer Promise as Infertility Treatment
A female mouse with synthetic ovaries created on a 3D-printer conceived and gave birth to healthy offspring. Researchers said the study could lead to an infertility treatment for women with cancer.
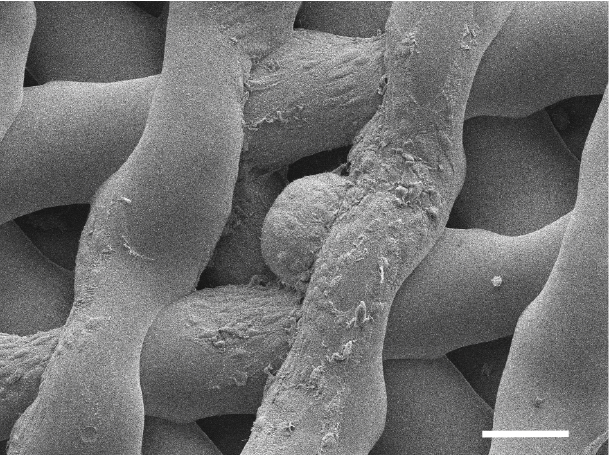
The ovary was created using a porous scaffold made from gelatin, according to the all-female research team, which described the study this week in the journal Nature Communications. Gelatin is a form of collagen, the most abundant protein in mammals. Compared to natural collagen, gelatin is more broken down and thus can be made into an ink that can be used in a 3D printer.
After making the gelatin scaffold, the researchers in the new study added ovarian cells taken from the ovary of another mouse. The ovarian cells form follicles that secrete hormones and release eggs.
"These ovarian cells are spherical. They are three-dimensional, and it's very critical that you maintain the right shape by giving them a three-dimensional structure" to live in, said Alexandra Rutz, one of the lead authors of the study, who participated in the project during her biomedical engineering graduate fellowship at Northwestern University Feinberg School of Medicine in Chicago. [5 Myths About Fertility Treatments]
"That's where the scaffold comes in. It has pores in it like a sponge," Rutz said. The pores can have different shapes, and the researchers found that one particular shape best supports the ovarian follicles. "Supporting their shape keeps them alive, and that keeps them functional," she told Live Science.
The ovaries are referred to as "biosynthetic" organs because they contain both living material (ovarian cells) and nonliving material (gelatin). They release hormones in the same way a normal ovary would, allowing the animal to go through its natural cycle, including ovulation, the researchers said. Because gelatin is a natural material, the body recognizes the implant as a regular body part and allows blood vessels to grow into it.
"The biosynthetic ovary was implanted into the exact same place where we removed the originally ovary from," Rutz said. "As the vessels grew into it, they naturally started picking up the hormones secreted by the ovarian cells and distributing them throughout the body to the target organs."
Get the world’s most fascinating discoveries delivered straight to your inbox.
Rutz said that the most challenging aspect of the study was designing the pores of the ovarian scaffold so they could properly support the ovarian cells in the long term.
The team is now preparing a similar trial on pigs, which are closer to humans in size and biology. Rutz said that scaling up the 3D-printed structure to the size needed for human use might be a challenge.
"In humans, the ovarian follicles can be as large as 15 millimeters [0.6 inches], which is huge, so we have to make sure that the scaffold design can actually accommodate such large cells," Rutz explained. "But we also have to look at the long-term performance of these implants in order to be able to provide an ovary replacement with a lifelong function that would last for years." [5 Things Women Should Know About Ovarian Cancer]
One day, such implants could be life-changing for women with impaired ovaries, the scientists said. The researchers added that they hope, in particular, to help survivors of childhood cancer whose cancer treatments damaged their ovaries.
"Their ovaries don't function at a high enough level, and they need to use hormone-replacement therapies in order to trigger puberty," said Monica Laronda, another lead author of the paper and a former postdoctoral fellow at Northwestern. "The purpose of this scaffold is to recapitulate how an ovary would function. We're thinking big picture, meaning every stage of the girl's life — so puberty, through adulthood, to a natural menopause."
Rutz said that in the future, such implants could help women who have impaired ovaries to conceive, and could naturally alleviate women's symptoms of menopause. Instead of having to use synthetic hormones that can cause unpleasant side effects, a woman could have an all-natural source of female hormones implanted directly into her body, Rutz said.
Originally published on Live Science.


